
Summary
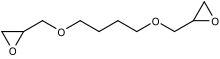
1,4-Butanediol diglycidyl ether (B14DODGE) is an organic chemical in the glycidyl ether family. It is aliphatic and a colorless liquid. It has two epoxide (oxirane) groups per molecule.[1] Its main use is in modifying epoxy resins especially viscosity reduction.[2]
| |
| Names | |
|---|---|
| IUPAC name
2-[4-(Oxiran-2-ylmethoxy)butoxymethyl]oxirane
| |
Other names
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.017.611 |
| EC Number |
|
| KEGG |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H18O4 | |
| Molar mass | 202.250 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
It is REACH registered.[3] The IUPAC name is 2-[4-(oxiran-2-ylmethoxy)butoxymethyl]oxirane.
Synthesis edit
1,4-Butanediol and epichlorohydrin are reacted in the presence of a Lewis acid as catalyst to form a halohydrin: each hydroxyl group of the diol reacts with an epoxide on epichlorohydrin. This process is followed by washing with sodium hydroxide to re-form the epoxide rings in dehydrochlorination reaction.[4] One of the quality control tests would involve measuring the Epoxy value by determination of the epoxy equivalent weight.
Uses edit
A key use is modifying the viscosity and properties of epoxy resins[5] which may then be formulated into CASE applications: Coatings,[6] Adhesives, Sealants, Elastomers, composite materials, fillers, putties, plasters, modelling clay and semiconductors. It also has a number of medical applications.[7][8][9] The molecule is also used to synthesize other molecules.[10][11] As an Epoxy modifier it is classed as an epoxy reactive diluent.The use of the diluent does effect mechanical properties and microstructure of epoxy resins.[12][13]
Toxicity edit
The toxicity is fairly well known and understood and is rated as a severe skin and eye irritant. Contact dermatitis is also possible.[14][15][16]
References edit
- ^ PubChem. "1,4-Butanediol diglycidyl ether". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-04-02.
- ^ Jagtap, Ameya Rajendra; More, Aarti (2022-08-01). "Developments in reactive diluents: a review". Polymer Bulletin. 79 (8): 5667–5708. doi:10.1007/s00289-021-03808-5. ISSN 1436-2449. S2CID 235678040.
- ^ "Substance Information - ECHA". echa.europa.eu. Retrieved 2022-04-02.
- ^ Crivello, James V. (2006). "Design and synthesis of multifunctional glycidyl ethers that undergo frontal polymerization". Journal of Polymer Science Part A: Polymer Chemistry. 44 (21): 6435–6448. Bibcode:2006JPoSA..44.6435C. doi:10.1002/pola.21761. ISSN 0887-624X.
- ^ Monte, Salvatore J. (1998), Pritchard, Geoffrey (ed.), "Diluents and viscosity modifiers for epoxy resins", Plastics Additives: An A-Z reference, Polymer Science and Technology Series, vol. 1, Dordrecht: Springer Netherlands, pp. 211–216, doi:10.1007/978-94-011-5862-6_24, ISBN 978-94-011-5862-6, retrieved 2022-03-29
- ^ Howarth G.A "Synthesis of a legislation compliant corrosion protection coating system based on urethane, oxazolidine and waterborne epoxy technology" page 23 Master of Science Thesis April 1997 Imperial College London
- ^ Ji, Gyu Yeul; Oh, Chang Hyun; Moon, Byung Gwan; Yi, Seong; Han, In Bo; Heo, Dong Hwa; Kim, Ki-Tack; Shin, Dong Ah; Kim, Keung Nyun (June 2015). "Efficacy and Safety of Sodium Hyaluronate with 1,4-Butanediol Diglycidyl Ether Compared to Sodium Carboxymethylcellulose in Preventing Adhesion Formation after Lumbar Discectomy". Korean Journal of Spine. 12 (2): 41–47. doi:10.14245/kjs.2015.12.2.41. ISSN 1738-2262. PMC 4513167. PMID 26217381.
- ^ Nicoletti, A.; Fiorini, M.; Paolillo, J.; Dolcini, L.; Sandri, M.; Pressato, D. (2013-01-01). "Effects of different crosslinking conditions on the chemical–physical properties of a novel bio-inspired composite scaffold stabilised with 1,4-butanediol diglycidyl ether (BDDGE)". Journal of Materials Science: Materials in Medicine. 24 (1): 17–35. doi:10.1007/s10856-012-4782-4. ISSN 1573-4838. PMID 23053811. S2CID 22093094.
- ^ Fiorani, Andrea; Gualandi, Chiara; Panseri, Silvia; Montesi, Monica; Marcacci, Maurilio; Focarete, Maria Letizia; Bigi, Adriana (2014-10-01). "Comparative performance of collagen nanofibers electrospun from different solvents and stabilized by different crosslinkers". Journal of Materials Science: Materials in Medicine. 25 (10): 2313–2321. doi:10.1007/s10856-014-5196-2. ISSN 1573-4838. PMID 24664673. S2CID 5270837.
- ^ Wu, Chi; Zuo, Ju; Chu, Benjamin (February 1989). "Molecular weight distribution of a branched epoxy polymer: 1,4-butanediol diglycidyl ether with cis-1,2-cyclohexanedicarboxylic anhydride". Macromolecules. 22 (2): 633–639. Bibcode:1989MaMol..22..633W. doi:10.1021/ma00192a021. ISSN 0024-9297.
- ^ Xue, Yu; Chen, Hongyue; Xu, Chao; Yu, Dinghua; Xu, Huajin; Hu, Yi (2020). "Synthesis of hyaluronic acid hydrogels by crosslinking the mixture of high-molecular-weight hyaluronic acid and low-molecular-weight hyaluronic acid with 1,4-butanediol diglycidyl ether". RSC Advances. 10 (12): 7206–7213. Bibcode:2020RSCAd..10.7206X. doi:10.1039/C9RA09271D. PMC 9049836. PMID 35493875. S2CID 214083413.
- ^ Khalina, Morteza; Beheshty, Mohammad Hosain; Salimi, Ali (2019-08-01). "The effect of reactive diluent on mechanical properties and microstructure of epoxy resins". Polymer Bulletin. 76 (8): 3905–3927. doi:10.1007/s00289-018-2577-6. ISSN 1436-2449. S2CID 105389177.
- ^ Pastarnokienė, Liepa; Jonikaitė-Švėgždienė, Jūratė; Lapinskaitė, Neringa; Kulbokaitė, Rūta; Bočkuvienė, Alma; Kochanė, Tatjana; Makuška, Ričardas (2023-07-01). "The effect of reactive diluents on curing of epoxy resins and properties of the cured epoxy coatings". Journal of Coatings Technology and Research. 20 (4): 1207–1221. doi:10.1007/s11998-022-00737-4. ISSN 1935-3804. S2CID 256749849.
- ^ "1,4-Butanediol diglycidyl ether - Hazardous Agents | Haz-Map". haz-map.com. Retrieved 2022-04-02.
- ^ Jolanki, Riitta; Estlander, Tuula; Kanerva, Lasse (February 1987). "Contact allergy to an epoxy reactive diluent: 1,4-butanediol diglycidyl ether". Contact Dermatitis. 16 (2): 87–92. doi:10.1111/j.1600-0536.1987.tb01385.x. PMID 2952443. S2CID 36846087 – via Wiley online.
- ^ Berdasco, Nancy Anne M.; Waechter, John M. (2012-08-17), Bingham, Eula; Cohrssen, Barbara; Powell, Charles H. (eds.), "Epoxy Compounds: Aromatic Diglycidyl Ethers, Polyglycidyl Ethers, Glycidyl Esters, and Miscellaneous Epoxy Compounds", Patty's Toxicology, Hoboken, NJ, USA: John Wiley & Sons, Inc., pp. 491–528, doi:10.1002/0471435139.tox083.pub2, ISBN 978-0-471-12547-1, retrieved 2022-07-28
Further reading edit
- Epoxy resin technology. Paul F. Bruins, Polytechnic Institute of Brooklyn. New York: Interscience Publishers. 1968. ISBN 0-470-11390-1. OCLC 182890.
{{cite book}}: CS1 maint: others (link) - Flick, Ernest W. (1993). Epoxy resins, curing agents, compounds, and modifiers : an industrial guide. Park Ridge, NJ. ISBN 978-0-8155-1708-5. OCLC 915134542.
{{cite book}}: CS1 maint: location missing publisher (link) - Lee, Henry (1967). Handbook of epoxy resins. Kris Neville ([2nd, expanded work] ed.). New York: McGraw-Hill. ISBN 0-07-036997-6. OCLC 311631322.
- "Dow Epoxy Resins" (PDF).
External websites edit
- Hexion difunctional epoxy diluents
- Denacol epoxy diluent range
- Cargill Reactive diluents


