
Summary
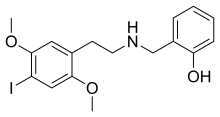
25I-NBOH (NBOH-2CI, Cimbi-27, 2-C-I-NBOH) is a derivative of the phenethylamine-derived hallucinogen 2C-I that was discovered in 2006 by a team at Purdue University.
 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID |
|
| ChemSpider |
|
| UNII |
|
| CompTox Dashboard (EPA) |
|
| Chemical and physical data | |
| Formula | C17H20INO3 |
| Molar mass | 413.255 g·mol−1 |
| 3D model (JSmol) |
|
| |
| |
| | |
Pharmacology edit
25I-NBOH acts as a potent agonist of the 5HT2A receptor,[2][3] with a Ki of 0.061 nM at the human 5HT2A receptor, similar to the better-known compound 25I-NBOMe, making it some twelve times the potency of 2C-I itself.
Although in vitro tests show this compound acts as an agonist, animal studies to confirm these findings have not been reported. While the N-benzyl derivatives of 2C-I had significantly increased binding to 5HT2A receptor fragments, compared to 2C-I, the N-benzyl derivatives of DOI were less active compared to DOI.[4]
25I-NBOH is notable as one of the most selective agonist ligands for the 5-HT2A receptor with an EC50 value of 0.074 nM with more than 400 times selectivity over the 5-HT2C receptor.[5]
Analytical chemistry edit
25I-NBOH is a labile molecule which fragments into 2C-I when analyzed by routine gas chromatography (GC) methods.[6] A specific method for reliable identification of 25I-NBOH using GC/MS has been reported, allowing forensic forces worldwide to correctly identify this compound.[7]
Legality edit
Sweden edit
The Riksdag added 25I-NBOH to Narcotic Drugs Punishments Act under Swedish schedule I ("substances, plant materials and fungi which normally do not have medical use") as of August 18, 2015, published by Medical Products Agency MPA) in regulation HSLF-FS 2015:12 listed as "25I-NBOH" and "2-([2-(4-jodo-2,5-dimetoxifenyl)etylamino]metyl)fenol".[8]
United Kingdom edit
This substance is a Class A drug in the United Kingdom as a result of the N-benzylphenethylamine catch-all clause in the Misuse of Drugs Act 1971.[9]
Analogues and derivatives edit
Analogues and derivatives of 2C-I:
25I-NB*:
- 25I-NBF
- 25I-NBMD
- 25I-NB34MD
- 25I-NBOH
- 25I-NBOMe (NBOMe-2CI)
- 25I-NB3OMe
- 25I-NB4OMe
- N-(2C-I) fentanyl[10]
References edit
- ^ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- ^ Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, et al. (April 2011). "Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT (2A) agonist PET tracers". European Journal of Nuclear Medicine and Molecular Imaging. 38 (4): 681–93. doi:10.1007/s00259-010-1686-8. PMID 21174090. S2CID 12467684.
- ^ Silva ME, Heim R, Strasser A, Elz S, Dove S (January 2011). "Theoretical studies on the interaction of partial agonists with the 5-HT2A receptor". Journal of Computer-Aided Molecular Design. 25 (1): 51–66. Bibcode:2011JCAMD..25...51S. CiteSeerX 10.1.1.688.2670. doi:10.1007/s10822-010-9400-2. PMID 21088982. S2CID 3103050.
- ^ Braden MR, Parrish JC, Naylor JC, Nichols DE (December 2006). "Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists". Molecular Pharmacology. 70 (6): 1956–64. doi:10.1124/mol.106.028720. PMID 17000863. S2CID 15840304.
- ^ Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL (March 2014). "Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists". ACS Chemical Neuroscience. 5 (3): 243–9. doi:10.1021/cn400216u. PMC 3963123. PMID 24397362.
- ^ Arantes LC, Júnior EF, de Souza LF, Cardoso AC, Alcântara TL, Lião LM, et al. (2017). "2A receptor agonist identified in blotter paper seizures in Brazil". Forensic Toxicology. 35 (2): 408–414. doi:10.1007/s11419-017-0357-x. PMC 5486617. PMID 28706567.
- ^ Neto JC, Andrade AF, Lordeiro RA, Machado Y, Elie M, Júnior EF, Arantes LC (2017). "Preventing misidentification of 25I-NBOH as 2C-I on routine GC–MS analyses" (PDF). Forensic Toxicology. 35 (2): 415–420. doi:10.1007/s11419-017-0362-0. S2CID 32432586.
- ^ "Gemensamma författningssamlingen avseende hälso- och sjukvård, socialtjänst, läkeme del, folkhälsa m.m." (PDF). Lakemedelsverket. Archived from the original (PDF) on 2017-10-30. Retrieved 2017-04-21.
- ^ "The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014". UK Statutory Instruments 2014 No. 1106. www.legislation.gov.uk.
- ^ "Explore N-(2C-I)-Fentanyl | PiHKAL · info". isomerdesign.com.


