
Summary
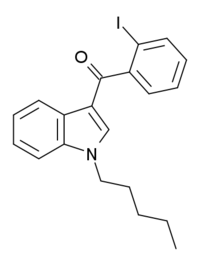
AM-679 (part of the AM cannabinoid series) is a drug that acts as a moderately potent agonist for the cannabinoid receptors, with a Ki of 13.5 nM at CB1 and 49.5 nM at CB2.[1] AM-679 was one of the first 3-(2-iodobenzoyl)indole derivatives that was found to have significant cannabinoid receptor affinity, and while AM-679 itself has only modest affinity for these receptors, it was subsequently used as a base to develop several more specialised cannabinoid ligands that are now widely used in research, including the potent CB1 agonists AM-694 and AM-2233, and the selective CB2 agonist AM-1241.[2] AM-679 was first identified as having been sold as a cannabinoid designer drug in Hungary in 2011, along with another novel compound 1-pentyl-3-(1-adamantoyl)indole.[3]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| ChemSpider |
|
| UNII |
|
| CompTox Dashboard (EPA) |
|
| Chemical and physical data | |
| Formula | C20H20INO |
| Molar mass | 417.290 g·mol−1 |
| 3D model (JSmol) |
|
| |
| |
| | |
See also edit
References edit
- ^ WO patent 200128557, Makriyannis A, Deng H, "Cannabimimetic indole derivatives", granted 2001-06-07
- ^ Deng H (2000). Design and synthesis of selective cannabinoid receptor ligands: Aminoalkylindole and other heterocyclic analogs (PhD Dissertation). University of Connecticut. ProQuest 304624325.
- ^ Jankovics P, Váradi A, Tölgyesi L, Lohner S, Németh-Palotás J, Balla J (January 2012). "Detection and identification of the new potential synthetic cannabinoids 1-pentyl-3-(2-iodobenzoyl)indole and 1-pentyl-3-(1-adamantoyl)indole in seized bulk powders in Hungary". Forensic Science International. 214 (1–3): 27–32. doi:10.1016/j.forsciint.2011.07.011. PMID 21813254.


