
KNOWPIA
WELCOME TO KNOWPIA
Summary
Acepentalene is a tricyclic anti-aromatic compound. Its molecular formula is C10H6. It consists of three five-membered rings fused across three of the five carbon atoms. The central carbon atom in acepentalene is part of all three rings. There are formally five double bonds in acepentalene, so that the molecule formally contains four double bonds on the exterior, and one double bond from the central carbon to the exterior of the ring system.
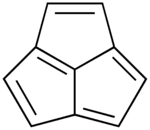
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Cyclopenta[cd]pentalene | |
| Other names
Acepentylene
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H6 | |
| Molar mass | 126.158 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
The acepentalene dianion, which can be stabilized by two lithium atoms, is more stable. The radical anion is also known.[1]
The dianion was first synthesized by reacting triquinacene with n-butyllithium and potassium tert-amylate (also called potassium t-pentoxide) in hexane solution.[2][3]
References edit
- ^ Armin de Meijere; Fabian Gerson; Peter R. Schreiner; Pascal Merstetter; Franz-Manfred Schüngel (1999). "The radical anion of acepentalene". Chem. Commun. (21): 2189–2190. doi:10.1039/a906972k.
- ^ Lendvai, Thomas; Friedl, Thomas; Butenschön, Holger; Clark, Timothy; Meijere, Armin de (1986). "Dihydroacepentalenediide, the Dianion of Acepentalene". Angewandte Chemie International Edition in English. 25 (8): 719–720. doi:10.1002/anie.198607191. ISSN 1521-3773.
- ^ Haag, Rainer; Kuck, Dietmar; Fu, Xiao-Yong; Cook, James W.; DeMeijere, Armin (1994). "Facile Formation of Dihydroacepentalenediide from centro-Substituted Tribenzotriquinacenes with C-C Bond Cleavage". Synlett. 1994 (5): 340–342. doi:10.1055/s-1994-22846. ISSN 0936-5214. S2CID 196822833.


