
KNOWPIA
WELCOME TO KNOWPIA
Summary
Aleglitazar is a peroxisome proliferator-activated receptor agonist (hence a PPAR modulator ) with affinity to PPARα and PPARγ, which was under development by Hoffmann–La Roche for the treatment of type II diabetes.[1] It is no longer in phase III clinical trials.[2]
| |
| Names | |
|---|---|
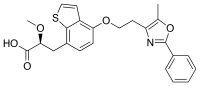
| Preferred IUPAC name
(2S)-2-Methoxy-3-{4-[2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)ethoxy]-1-benzothiophen-7-yl}propanoic acid | |
| Other names
Ro-0728804, R-1439
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEMBL |
|
| ChemSpider |
|
| DrugBank |
|
| ECHA InfoCard | 100.220.523 |
| |
| KEGG |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C24H23NO5S | |
| Molar mass | 437.51 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
References edit


