
Summary
Avibactam is a non-β-lactam β-lactamase inhibitor[2] developed by Actavis (now Teva) jointly with AstraZeneca. A new drug application for avibactam in combination with ceftazidime (branded as Avycaz) was approved by the FDA on February 25, 2015, for treating complicated urinary tract (cUTI) and complicated intra-abdominal infections (cIAI) caused by antibiotic resistant-pathogens, including those caused by multi-drug resistant Gram-negative bacterial pathogens.[3][4][5]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Avycaz (formulated with ceftazidime) |
| Routes of administration | Intravenous therapy |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100% (intravenous) |
| Protein binding | 5.7–8.2%[1] |
| Metabolism | Nil |
| Onset of action | Increases in proportion to dose |
| Excretion | Kidney (97%) |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| Chemical and physical data | |
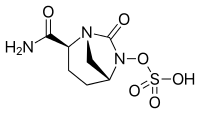
| Formula | C7H11N3O6S |
| Molar mass | 265.24 g·mol−1 |
| 3D model (JSmol) |
|
| |
| |
Increasing resistance to cephalosporins among Gram-(−) bacterial pathogens, especially among hospital-acquired infections, results in part from the production of β-lactamase enzymes that deactivate these antibiotics. While the co-administration of a β-lactamase inhibitor can restore antibacterial activity to the cephalosporin, previously approved β-lactamase inhibitors such as tazobactam and clavulanic acid do not inhibit important classes of β-lactamases, including Klebsiella pneumoniae carbapenemases (KPCs), New Delhi metallo-β-lactamase 1 (NDM-1), and AmpC-type β-lactamases. Whilst avibactam inhibits class A (KPCs, CTX-M, TEM, SHV), class C (AmpC), and, some, class D serine β-lactamases (such as OXA-23, OXA-48), it has been reported to be a poor substrate/weak inhibitor of class B metallo-β-lactamases, such as VIM-2, VIM-4, SPM-1, BcII, NDM-1, Fez-1.[6]
For infections sustained by metallo-β-lactamases producing bacteria, a therapeutic strategy consists in administering avibactam as companion drug administered alongside aztreonam. In fact, although in theory aztreonam is not hydrolyzed by metallo-β-lactamases, many metallo-β-Lactamases-producing strains co-produce enzymes that could hydrolyze aztreonam (e.g. AmpC, ESBL), therefore avibactam is given to protect aztreonam exploiting its robust β-lactamases inhibition.[7] Avibactam is available in a combination with aztreonam (aztreonam/avibactam).
References edit
- ^ "Full Prescribing Information: Avycaz (ceftazidime-avibactam) for Injection, for intravenous use". ©2015 Actavis. All rights reserved. Archived from the original on 2 June 2015. Retrieved 1 June 2015.
- ^ Wang DY, Abboud MI, Markoulides MS, Brem J, Schofield CJ (June 2016). "The road to avibactam: the first clinically useful non-β-lactam working somewhat like a β-lactam". Future Medicinal Chemistry. 8 (10): 1063–1084. doi:10.4155/fmc-2016-0078. PMID 27327972.
- ^ Zhanel GG, Lawson CD, Adam H, Schweizer F, Zelenitsky S, Lagacé-Wiens PR, et al. (February 2013). "Ceftazidime-avibactam: a novel cephalosporin/β-lactamase inhibitor combination". Drugs. 73 (2): 159–177. doi:10.1007/s40265-013-0013-7. PMID 23371303. S2CID 32700350.
- ^ "Actavis Announces FDA Acceptance of the NDA Filing for Ceftazidime-Avibactam, a Qualified Infectious Disease Product". Actavis—a global, integrated specialty pharmaceutical company—Actavis. Actavis plc. Archived from the original on 27 May 2015. Retrieved 1 June 2015.
- ^ Ehmann DE, Jahic H, Ross PL, Gu RF, Hu J, Durand-Réville TF, et al. (September 2013). "Kinetics of avibactam inhibition against Class A, C, and D β-lactamases". The Journal of Biological Chemistry. 288 (39): 27960–27971. doi:10.1074/jbc.M113.485979. PMC 3784710. PMID 23913691.
- ^ Abboud MI, Damblon C, Brem J, Smargiasso N, Mercuri P, Gilbert B, et al. (October 2016). "Interaction of Avibactam with Class B Metallo-β-Lactamases". Antimicrobial Agents and Chemotherapy. 60 (10): 5655–5662. doi:10.1128/AAC.00897-16. PMC 5038302. PMID 27401561.
- ^ Mauri C, Maraolo AE, Di Bella S, Luzzaro F, Principe L (August 2021). "The Revival of Aztreonam in Combination with Avibactam against Metallo-β-Lactamase-Producing Gram-Negatives: A Systematic Review of In Vitro Studies and Clinical Cases". Antibiotics. 10 (8): 1012. doi:10.3390/antibiotics10081012. PMC 8388901. PMID 34439062.
Further reading edit
- Edeki T, Armstrong J, Li J (September 2013). Pharmacokinetics of avibactam (AVI) and ceftazidime (CAZ) following separate or combined administration in healthy volunteers. 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC). Vol. 10. p. 13. Poster A-1019. Archived from the original on 2016-03-03.


