
Summary
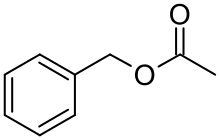
Benzyl acetate is an organic ester with the molecular formula CH3C(O)OCH2C6H5. It is formed by the condensation of benzyl alcohol and acetic acid.
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Benzyl acetate | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider |
|
| ECHA InfoCard | 100.004.909 |
| KEGG |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CH3C(O)OCH2C6H5 | |
| Molar mass | 150.18 g/mol |
| Appearance | Colourless liquid |
| Odor | floral |
| Density | 1.054 g/ml |
| Melting point | −51.5 °C (−60.7 °F; 221.7 K) |
| Boiling point | 212 °C (414 °F; 485 K) |
| 0.31 g/100 mL | |
| Solubility | Soluble in benzene, chloroform Miscible with ethanol, ether, acetone |
| -93.18·10−6 cm3/mol | |
Refractive index (nD)
|
1.523 |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 102 °C (216 °F; 375 K) |
| 461 °C (862 °F; 734 K) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Similar to most other esters, it possesses a sweet and pleasant aroma, owing to which, it finds applications in personal hygiene and health care products. It is a constituent of jasmin and of the essential oils of ylang-ylang and neroli. It has pleasant sweet aroma reminiscent of jasmine. Further as a flavoring agent it is also used to impart jasmine or apple flavors to various cosmetics and personal care products like lotions, hair creams etc..[1]
It is one of many compounds that is attractive to males of various species of orchid bees. It is collected and used by the bees as an intra-specific pheromone; In apiculture benzyl acetate is used as a bait to collect bees. Natural sources of benzyl acetate include varieties of flowers like jasmine (Jasminum), and fruits like pear, apple, etc.[2]
References edit
External links edit
- International Chemical Safety Card 1331



