
Summary
In evolutionary biology, the term cellularization (cellularisation) has been used in theories to explain the evolution of cells, for instance in the pre-cell theory,[1][2][3] dealing with the evolution of the first cells on this planet, and in the syncytial theory[4] attempting to explain the origin of Metazoa from unicellular organisms.
Processes of cell development in multinucleate cells (syncytium, plural syncytia) of animals and plants are also termed cellularization, often called syncytium cellularization.
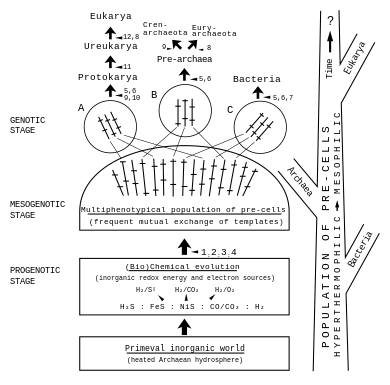
Key:
1 Reductive formation of organic compounds from CO or CO2 by Me-sulfur coordinative chemistry
2 tapping of various redox energy sources and formation of primitive enzymes and templates
3 elements of a transcription and translation apparatus and loose associations
4 formation of pre-cells
5 stabilised circular or linear genomes
6 cytoplasmic membranes
7 rigid murein cell walls
8 various non-murein rigid cell walls
9 glycoproteinaceous cell envelope or glycokalyx
10 cytoskeleton
11 complex chromosomes and nuclear membrane
12 cell organelles via endosymbiosis".
The pre-cell theory edit
According to Otto Kandler's pre-cell theory,[1][2][3] early evolution of life and primordial metabolism (see Iron-Sulfur world hypothesis - metabolism first scenario, according to Wächtershäuser[5][6]) led to the early diversification of life through the evolution of a multiphenotypical population of pre-cells,[1][2][3] from which the three founder groups A, B, C and then, from them, the precursor cells (here named proto-cells) of the three domains of life[7] emerged successively.[1][2][3][8]
In this scenario the three domains of life did not originate from an ancestral nearly complete “first cell“ nor a cellular organism often defined as the last universal common ancestor (LUCA[9][10][11]), but from a population of evolving pre-cells. Kandler introduced the term cellularization for his concept of a successive evolution of cells by a process of evolutionary improvements.[1][2][3]
His concept may explain the quasi-random distribution of evolutionarily important features among the three domains and, at the same time, the existence of the most basic biochemical features (genetic code, set of protein amino acids etc.) in all three domains (unity of life), as well as the close relationship between the Archaea and the Eucarya. Kandler’s pre-cell theory is supported by Wächtershäuser.[8]
According to Kandler, the protection of fragile primordial life forms from their environment by the invention of envelopes (i.e. membranes, walls) was an essential improvement. For instance, the emergence of rigid cell walls by the invention and elaboration of peptidoglycan[12] in bacteria (domain Bacteria) may have been a prerequisite for their successful survival, radiation and colonisation of virtually all habitats of the geosphere and hydrosphere.[3]
A coevolution of the biosphere and the geosphere is suggested: “The evolving life could venture into a larger variety of habitats, even into microaerobic habitats in shallow, illuminated surface waters. The continuous changes in the physical environment on the aging and cooling Earth led to further diversification of habitats and favored opportunistic radiation of primitive life into numerous phenotypes on the basis of each of the different chemolithoautotrophies. Concomitantly, with the accumulation of organic matter derived from chemolithoautotrophic life, opportunistic and obligate heterotrophic life may also have developed”.[1]: 155f
The details of Kandler's proposal for the early diversification of life are represented in a scheme, where numbers indicate evolutionary improvements.[3]
The syncytial theory or ciliate-acoel theory edit
This theory is also known as a theory of cellularization. It is a theory to explain the origin of the Metazoa. The idea was proposed by Hadži (1953)[4] and Hanson (1977).[13]
This cellularization (syncytial) theory states that metazoans evolved from a unicellular ciliate with multiple nuclei that went through cellularization. Firstly, the ciliate developed a ventral mouth for feeding and all nuclei moved to one side of the cell. Secondly, an epithelium was created by membranes forming barriers between the nuclei. In this way, a multicellular organism was created from one multinucleate cell (syncytium).[14]
Example and Criticism edit
Turbellarian flatworms edit
According to the syncytial theory, the ciliate ancestor, by several cellularization processes, evolved into the currently known turbellarian flatworms, which are therefore the most primitive metazoans. The theory of cellularization is based on the large similarities between ciliates and flatworms. Both ciliates and flatworms have cilia, are bilaterally symmetric, and syncytial. Therefore, the theory assumes that bilateral symmetry is more primitive than radial symmetry. However, current biological evidence shows that the most primitive forms of metazoans show radial symmetry, and thus radially symmetrical animals like cnidaria cannot be derived from bilateral flatworms.[15]
By concluding that the first multicellular animals were flatworms, it is also suggested that simpler organisms as sponges, ctenophores and cnidarians would have derived from more complex animals.[16] However, most current molecular research has shown that sponges are the most primitive metazoans.[17][18]
Germ layers are formed simultaneously edit
The syncytial theory rejects the theory of germ layers. During the development of the turbellaria (Acoela), three regions are formed without the formation of germ layers. From this, it was concluded that the germ layers are simultaneously formed during the cellularization process. This is in contrast to germ layer theory in which ectoderm, endoderm and mesoderm (in more complex animals) build up the embryo.[19]
The macro and micronucleus of Ciliates edit
There is a lot of evidence against ciliates being the metazoan ancestor. Ciliates have two types of nuclei: a micronucleus which is used as germline nucleus and a macronucleus which regulates the vegetative growth.[20] This division of nuclei is a unique feature of the ciliates and is not found in any other members of the animal kingdom.[21] Therefore, it would be unlikely that ciliates are indeed the ancestors of the metazoans. This is confirmed by molecular phylogenetic research. Ciliates were never found close to animals in any molecular phylogeny.[22]
Flagellated sperm edit
Furthermore, the syncytial theory cannot explain the flagellated sperm of metazoans. Since the ciliate ancestor does not have any flagella and it is unlikely that the flagella arose as a de novo trait in metazoans, the syncytial theory makes it almost impossible to explain the origin of flagellated sperm.[19]
Due to both the lack of molecular and morphological evidence for this theory, the alternative colonial theory of Haeckel, is currently gaining widespread acceptance.
For more theories see main article Multicellular organisms.
Cellularization in a syncytium (syncytium cellularization) edit
The development of cells in a syncytium (multinucleate cells) is termed syncytium cellularization. Syncytia are quite frequent in animals and plants. Syncytium cellularization occurs for instance in the embryonic development of animals and in endosperm development of plants. Here two examples:
Drosophila melanogaster development edit
In the embryonic development of Drosophila melanogaster, first 13 nuclear divisions take place forming a syncytial blastoderm consisting of approximately 6000 nuclei. During the later gastrulation stage, membranes are formed between the nuclei, and cellularization is completed.[23]
Syncytium cellularization in plants edit
The term syncytium cellularization is used for instance for a process of cell development in the endosperm of the Poaceae, e.g. barley (Hordeum vulgare),[24] rice (Oryza sativa).[25]
See also edit
References edit
- ^ a b c d e f Kandler, Otto (1994). "The early diversification of life". In Stefan Bengtson (ed.). Early Life on Earth. Nobel Symposium 84. New York: Columbia U.P. pp. 152–160.
- ^ a b c d e Kandler, Otto (1995). "Cell Wall Biochemistry in Archaea and its Phylogenetic Implications". Journal of Biological Physics. 20 (1–4): 165–169. doi:10.1007/BF00700433. S2CID 83906865.
- ^ a b c d e f g h Kandler, Otto (1998). "The early diversification of life and the origin of the three domains: A proposal". In Jürgen Wiegel; Michael W.W. Adams (eds.). Thermophiles: The keys to molecular evolution and the origin of life?. London: Taylor and Francis Ltd. pp. 19–31. ISBN 978-0-203-48420-3.
- ^ a b Hadži, J. (1953-12-01). "An Attempt to Reconstruct the System of Animal Classification". Systematic Biology. 2 (4): 145–154. doi:10.2307/sysbio/2.4.145. ISSN 1063-5157.
- ^ Wächtershäuser, Günter (December 1988). "Before Enzymes and Templates: Theory of Surface Metabolism" (PDF). Microbiology and Molecular Biology Reviews. 52 (4): 452–484. doi:10.1128/mr.52.4.452-484.1988. PMC 373159. PMID 3070320.
- ^ Wächtershäuser, Günter (1998). "The Case for a Hyperthermophilic, Chemolithoautotrophic Origin of Life in an Iron-Sulfur World". In Jürgen Wiegel; Michael W.W. Adams (eds.). Thermophiles: The keys to molecular evolution and the origin of life?. London: Taylor and Francis Ltd. pp. 19–31. ISBN 978-0-203-48420-3.
- ^ Woese, Carl R.; Kandler, Otto; Wheelis, M. L. (June 1990). "Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya". Proceedings of the National Academy of Sciences of the United States of America. 87 (12): 4576–4579. Bibcode:1990PNAS...87.4576W. doi:10.1073/pnas.87.12.4576. PMC 54159. PMID 2112744.
- ^ a b Wächtershäuser, Günter (2003). "From pre-cells to Eukarya – a tale of two lipids". Molecular Microbiology. 47 (1): 13–22. doi:10.1046/j.1365-2958.2003.03267.x. PMID 12492850. S2CID 37944519.
- ^ Harold, Franklin M. (2014). In Search of Cell History: The Evolution of Life's Building Blocks. Chicago, London: University of Chicago Press. ISBN 978-0-226-17428-0.
- ^ Madigan, Michael T.; Martinko, John M.; Bender, Kelly S.; Buckley, Daniel H.; Stahl, David A. (2015). Brock Biology of Microorganisms (14 ed.). Boston: Pearson Education Limited. pp. 29, 374, 381. ISBN 978-1-292-01831-7.
- ^ Madigan, Michael T.; Aiyer, Jennifer; Buckley, Daniel H.; Sattley, Matthew; Stahl, David A. (2022). Brock Biology of Microorganisms (16 ed.). Harlow: Pearson Education. pp. 431, 435. ISBN 978-1-292-40479-0.
- ^ Schleifer, Karl-Heinz; Kandler, Otto (1972). "Peptidoglycan types of bacterial cell Walls and their taxonomic implications". Bacteriological Reviews. 36 (4): 407–775. doi:10.1128/br.36.4.407-477.1972. PMC 408328. PMID 4568761.
- ^ Hanson, Earl D. (1977). The origin and early evolution of animals (1st ed.). Middletown, Conn.: Wesleyan University Press. ISBN 0819550086. OCLC 2597099.
- ^ Klautau, M.; Russo, C.A.M. (2016), "Metazoans, Origins of", Encyclopedia of Evolutionary Biology, Elsevier, pp. 1–6, doi:10.1016/b978-0-12-800049-6.00270-5, ISBN 9780128004265
- ^ Pilato, Giovanni (2007). The origin and phylogeny of the metazoans and the theory of endoderm as secondary layer. Foxwell & Davies. ISBN 978-1905868063. OCLC 488084010.
- ^ Waggoner, Ben (2001-04-25), "Eukaryotes and Multicells: Origin", eLS, John Wiley & Sons, Ltd, doi:10.1038/npg.els.0001640, ISBN 0470016175
- ^ Schütze, Joachim; Krasko, Anatoli; Custodio, Marcio Reis; Efremova, Sofla M.; Müller, Isabel M.; Müller, Werner E. G. (1999-01-07). "Evolutionary relationships of Metazoa within the eukaryotes based on molecular data from Porifera". Proceedings of the Royal Society of London. Series B: Biological Sciences. 266 (1414): 63–73. doi:10.1098/rspb.1999.0605. ISSN 0962-8452. PMC 1689648. PMID 10081159.
- ^ Manuel, Michaël; Wörheide, Gert; Morgenstern, Burkhard; Erpenbeck, Dirk; Schreiber, Fabian; Jackson, Daniel J.; Leys, Sally; Guyader, Hervé Le; Wincker, Patrick (2009-04-28). "Phylogenomics Revives Traditional Views on Deep Animal Relationships". Current Biology. 19 (8): 706–712. doi:10.1016/j.cub.2009.02.052. ISSN 0960-9822. PMID 19345102.
- ^ a b R.L.Kotpal, Prof (2012). Modern Text Book of Zoology: Invertebrates. Rastogi Publications. ISBN 9788171339037.
- ^ Prescott, D M (June 1994). "The DNA of ciliated protozoa". Microbiological Reviews. 58 (2): 233–267. doi:10.1128/MMBR.58.2.233-267.1994. ISSN 0146-0749. PMC 372963. PMID 8078435.
- ^ Lipscomb, Diana (March 1991). "Protoctists Close at Hand Handbook of Protoctista L. Margulis J. O. Corliss M. Melkonian D. J. Chapman". BioScience. 41 (3): 169–170. doi:10.2307/1311459. ISSN 0006-3568. JSTOR 1311459.
- ^ Schlegel, Martin (September 1994). "Molecular phylogeny of eukaryotes". Trends in Ecology & Evolution. 9 (9): 330–335. doi:10.1016/0169-5347(94)90153-8. ISSN 0169-5347. PMID 21236876.
- ^ Campos-Ortega, Jose A.; Hartenstein, Volker (2013-11-11). The Embryonic Development of Drosophila melanogaster. Springer Science & Business Media. ISBN 9783662224892.
- ^ Journal Article The Dynamics of Transcript Abundance during Cellularization of Developing Barley Endosperm Runxuan Zhang, Matthew R. Tucker, Rachel A Burton, Neil J. Shirley, Alan Little, Jenny Morris, Linda Milne, Kelly Houston, Pete E. Hedley, Robbie Waugh, Geoffrey B. Fincher (2016). "The Dynamics of Transcript Abundance during Cellularization of Developing Barley Endosperm". Plant Physiology. 170 (3): 1549–1565. doi:10.1104/pp.15.01690.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Masanori Mizutani, Takuma Naganuma, Ken-ichi Tsutsumi, Yasushi Saitoh (2010). "The syncytium-specific expression of the Orysa;KRP3 CDK inhibitor: implication of its involvement in the cell cycle control in the rice (Oryza sativa L.) syncytial endosperm". Journal of Experimental Botany. 61 (3): 791–798. doi:10.1093/jxb/erp343. PMC 2814109. PMID 19933315.
{{cite journal}}: CS1 maint: multiple names: authors list (link)


