
KNOWPIA
WELCOME TO KNOWPIA
Summary
Cholane is a triterpene which can exist as either of two stereoisomers, 5α-cholane and 5β-cholane. Its name is derived from Greek: χολή (chole) meaning 'bile' in reference to its original discovery from the bile of the American bullfrog (Rana catesbeiana).[2] The compound itself has no known uses. However, various functionalized analogues are produced by plants and animals, typically in the form of sterols, steroids and bile acids (e.g. cholic acid).
-
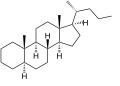 5α-Cholane
5α-Cholane -
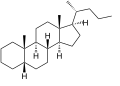 5β-Cholane
5β-Cholane
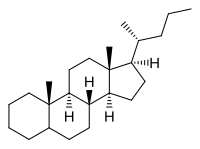
| |
| Names | |
|---|---|
| IUPAC name
Cholane[1]
| |
| Systematic IUPAC name
(1R,3aS,3bR,5aΞ,9aS,9bS,11aR)-9a,11a-Dimethyl-1-[(2R)-pentan-2-yl]hexadecahydro-1H-cyclopenta[a]phenanthrene | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C24H42 | |
| Molar mass | 330.59 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
See also edit
References edit
- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 1528. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ Kurauti, Yukiti; Kazuno, Taro (January 1939). "Tetraoxycholan, Trioxycholen und Trioxy-bis-norsterocholansäure aus der Galle von Rana Catesbina Shaw". Hoppe-Seyler's Zeitschrift für physiologische Chemie (in German). 262 (1–2): 53–60. doi:10.1515/bchm2.1939.262.1-2.53.
External links edit
- Cholanes at the U.S. National Library of Medicine Medical Subject Headings (MeSH)




