
KNOWPIA
WELCOME TO KNOWPIA
Summary
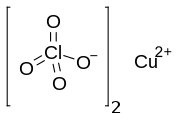
Copper(II) perchlorate is an inorganic compound with the chemical formula Cu(ClO4)2. It is a salt of copper and perchloric acid. It is a hygroscopic crystalline blue solid. It is commonly encountered as copper(II) perchlorate hexahydrate, According to X-ray crystallography, the salt is the aquo complex [Cu(H2O)6]2+ together with the weakly coordinating anion ClO−4.[4]
| |

| |
 Copper(II) perchlorate hexahydrate
| |
| Names | |
|---|---|
| IUPAC name
Copper(II) perchlorate
| |
| Other names
Cupric perchlorate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider |
|
| ECHA InfoCard | 100.033.978 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Cu(ClO4)2 | |
| Molar mass | 262.447 g/mol (anhydrous) 370.539 g/mol (hexahydrate) |
| Appearance | Blue crystalline hygroscopic solid (hexahydrate)[1] |
| Odor | odorless |
| Density | 2.225 g/cm3 (hexahydrate) |
| Melting point | 82 °C (180 °F; 355 K) (hexahydrate) |
| Boiling point | 120 °C (248 °F; 393 K) (hexahydrate) |
| 146 g/(100 ml) (30°C) | |
Refractive index (nD)
|
1.505 (hexahydrate)[2] |
| Hazards | |
| GHS labelling: | |
 
| |
| Warning | |
| H272, H315, H319, H335 | |
| P210, P220, P221, P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P370+P378, P403+P233, P405, P501 | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 1 mg/m3 (as Cu)[3] |
REL (Recommended)
|
TWA 1 mg/m3 (as Cu)[3] |
IDLH (Immediate danger)
|
TWA 100 mg/m3 (as Cu)[3] |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Safety edit
Like any perchlorate, it is a strong oxidizing agent.
References edit
- ^ "Copper perchlorate | Cl2CuO8 | ChemSpider".
- ^ "Copper(Ii) Perchlorate Hexahydrate | 10294-46-9".
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0150". National Institute for Occupational Safety and Health (NIOSH).
- ^ Gallucci, J. C.; Gerkin, R. E. (1989). "Structure of copper(II) perchlorate hexahydrate". Acta Crystallogr. C. 45: 1279–1284. doi:10.1107/S0108270189000818.


