
KNOWPIA
WELCOME TO KNOWPIA
Summary
Dysprosium(III) bromide is an inorganic compound of bromine and dysprosium, with the chemical formula of DyBr3.
| |
| Identifiers | |
|---|---|
| |
3D model (JSmol)
|
|
| ChemSpider |
|
| ECHA InfoCard | 100.034.933 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Appearance | colourless solid (anhydrous)[1] white solid (hexahydrate)[2] |
| Density | 5.8 g·cm−3[3] |
| Melting point | 881 °C (1,154 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Preparation edit
Dysprosium(III) bromide can be obtained by reacting dysprosium with bromine:[4]
- 2Dy + 3Br2 → 2DyBr3
Dysprosium bromide hexahydrate can be obtained by crystallization from its solution,[2] which can be heated with ammonium bromide in vacuum to obtain the anhydrous form.[1]
Dysprosium(III) oxide and aluminium bromide (in the form of Al2Br6 at a high temperature react a DyAl3Br12, which decomposes to dysprosium(III) bromide at a lower temperature:[5]
- Dy2O3 + Al2Br6 → Al2O3 + 2 DyBr3
Properties edit
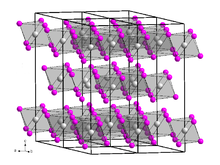
Dysprosium(III) bromide is a white-gray hygroscopic solid that is soluble in water.[6] It has a trigonal crystal structure of the bismuth(III) iodide type with space group R3 (No. 148).[7]
References edit
- ^ a b c Jantsch, G.; Jawurek, H.; Skalla, N.; Gawalowski, H. Halides of the rare earths. VI. Halides of the terbium and erbium earth groups. Zeitschrift fuer Anorganische und Allgemeine Chemie, 1932. 207. 353-367. ISSN 0044-2313.
- ^ a b D. Brown, S. Fletcher, D. G. Holah (1968). "The preparation and crystallographic properties of certain lanthanide and actinide tribromides and tribromide hexahydrates". Journal of the Chemical Society A: Inorganic, Physical, Theoretical: 1889–1894. doi:10.1039/j19680001889. ISSN 0022-4944. Retrieved 2020-05-29.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Roger Blachnik (Hrsg.): Taschenbuch für Chemiker und Physiker. Band III: Elemente, anorganische Verbindungen und Materialien, Minerale. begründet von Jean d’Ans, Ellen Lax. 4., neubearbeitete und revidierte Auflage. Springer, Berlin 1998, ISBN 3-540-60035-3, S. 442, 1386
- ^ WebElements: Chemical reactions of Dysprosium
- ^ 杨冬梅, 于锦, 蒋军辉,等. 化学气相传输法制备无水溴化镝. 石油化工高等学校学报, 2003, 16(4). doi: 10.3969/j.issn.1006-396X.2003.04.004.
- ^ Dysprosium(III) bromide, ultra dry, 99.99% (REO) at AlfaAesar, accessed on 2013-10-30 (PDF) (JavaScript required).[dead link]
- ^ Ans, Jean d'; Lax, Ellen (December 1997). Taschenbuch für Chemiker und Physiker. Springer. pp. 442, 1386. ISBN 3540600353.


