
KNOWPIA
WELCOME TO KNOWPIA
Summary
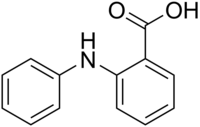
Fenamic acid is an organic compound, which, especially in its ester form, is called fenamate.[1]: 458 serves as a parent structure for several nonsteroidal anti-inflammatory drugs (NSAIDs), including mefenamic acid, tolfenamic acid, flufenamic acid, and meclofenamic acid. These drugs are commonly referred to as "anthranilic acid derivatives" or "fenamates" because fenamic acid is a derivative of anthranilic acid.[2]: 235 [3]: 17 [2]
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Anilinobenzoic acid | |
| Other names
N-phenylanthranilic acid
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.001.879 |
| |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H11NO2 | |
| Molar mass | 213.23 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Fenamic acid can be synthesized from 2-chlorobenzoic acid and can be converted into acridone.[4]
References edit
- ^ Gupta, PK. Drug NomenclatureUnited States Adopted Names. Ch 27 in Remington: The Science and Practice of Pharmacy, Vol 1. Eds. David B. Troy, Paul Beringer. Lippincott Williams & Wilkins, 2006 ISBN 9780781746731
- ^ a b Sriram D, Yogeeswari P. Medicinal Chemistry, 2nd Edition. Pearson Education India, 2010. ISBN 9788131731444
- ^ Auburn University course material. Jack DeRuiter, Principles of Drug Action 2, Fall 2002 1: Non-Steroidal Antiinflammatory Drugs (NSAIDS)
- ^ C. F. H. Allen, G. H. W. McKee (1939). "Acridone". Organic Syntheses. 2: 6. doi:10.15227/orgsyn.019.0006.


