Summary
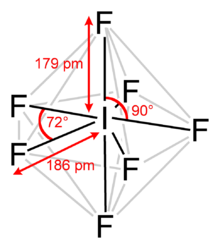
Iodine heptafluoride is an interhalogen compound with the chemical formula IF7.[2][3] It has an unusual pentagonal bipyramidal structure, with D5h symmetry, as predicted by VSEPR theory.[4] The molecule can undergo a pseudorotational rearrangement called the Bartell mechanism, which is like the Berry mechanism but for a heptacoordinated system.[5]
| |||
| |||
| Names | |||
|---|---|---|---|
| Other names
Iodine(VII) fluoride
Heptafluoroiodine | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
| ||
| ChemSpider |
| ||
| ECHA InfoCard | 100.037.241 | ||
PubChem CID
|
| ||
| UNII |
| ||
CompTox Dashboard (EPA)
|
| ||
| |||
| |||
| Properties | |||
| IF7 | |||
| Molar mass | 259.90 g/mol | ||
| Appearance | colorless gas | ||
| Density | 2.6 g/cm3 (6 °C) 2.7 g/cm3 (25 °C) | ||
| Melting point | 4.5 °C (40.1 °F; 277.6 K) (triple point) | ||
| Boiling point | 4.8 °C (40.6 °F; 277.9 K) (sublimes at 1 atm) | ||
| soluble [1] | |||
| Related compounds | |||
Related compounds
|
iodine pentafluoride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |||
Below 4.5 °C, IF7 forms a snow-white powder of colorless crystals, melting at 5-6 °C. However, this melting is difficult to observe, as the liquid form is thermodynamically unstable at 760 mmHg: instead, the compound begins to sublime at 4.77 °C. The dense vapor has a mouldy, acrid odour.[6][7]
Preparation edit
IF7 is prepared by passing F2 through liquid IF5 at 90 °C, then heating the vapours to 270 °C. Alternatively, this compound can be prepared from fluorine and dried palladium or potassium iodide to minimize the formation of IOF5, an impurity arising by hydrolysis.[8][9] Iodine heptafluoride is also produced as a by-product when dioxygenyl hexafluoroplatinate is used to prepare other platinum(V) compounds such as potassium hexafluoroplatinate(V), using potassium fluoride in iodine pentafluoride solution:[10]
- 2 O2PtF6 + 2 KF + IF5 → 2 KPtF6 + 2 O2 + IF7
Reactions edit
Iodine heptafluoride decomposes at 200 °C to fluorine gas and iodine pentafluoride.[11]
Safety considerations edit
IF7 is highly irritating to both the skin and the mucous membranes. It also is a strong oxidizer and can cause fire on contact with organic material.
References edit
- ^ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- ^ Macintyre, J. E. (Ed.). (1992). Dictionary of Inorganic Compounds (Vol. 3). London: Chapman & Hall.
- ^ O'Neil, Maryadele J. (Ed.). (2001). The Merck Index (13th ed.). Whitehouse Station, NJ: Merck.
- ^ K. O. Christe; E. C. Curtis; D. A. Dixon (1993). "On the problem of heptacoordination: vibrational spectra, structure, and fluxionality of iodine heptafluoride". Journal of the American Chemical Society. 115 (4): 1520–1526. doi:10.1021/ja00057a044.
- ^ W. J. Adams; H. Bradford Thompson; L. S. Bartell (1970). "Structure, Pseudorotation, and Vibrational Mode Coupling in IF7: An Electron Diffraction Study" (PDF). Journal of Chemical Physics. 53 (10): 4040–4046. Bibcode:1970JChPh..53.4040A. doi:10.1063/1.1673876. hdl:2027.42/71219.
- ^ Lide, David R. (2011). The CRC Handbook of Chemistry and Physics, 89th ed. p. 4-67.
- ^ Ruff & Keim 1930, pp. 180–182: "Das reine Jod-7-fluorid ist bei Zimmertemperatur ein farbloses Gas, das an der Luft infolge seiner Umsetzung mit dem Wasserdampf Nebel bildet und muffig sauer riecht. Beim Abkühlen wird es je nach den Versuchsbedingungen als leicht bewegliche, farblose Flüssigkeit als schneeweißes lockeres Pulver oder in Form farbloser Kristalle erhalten....Die Schmelztemperatur wurde durch Eintauchen von Proben, die in Quarzröhrchen eingeschmolzen waren, in Bäder passender Temperatur ermittelt und zu 5 bis 6° C gefunden. [Durch] Messung der Dampfdrucke...gelangt man zu der Gleichung ....Für den Druck von 760 mm errechnet sich eine Temperatur von 4,5° C. [Pure iodine heptafluoride is at room temperature a colorless gas, which forms a fog in air on account of its reaction with the humidity and smells musty and acrid. When cooled, it takes the form, depending on experimental conditions, of a free-flowing colorless liquid, a loose snow-white powder, or colorless crystals....The melting point was measured by immersion of a probe in a bath of the right temperature, which was melting in a little quartz tube and found to be 5‑6 °C. [From] measurement of the vapor pressure...one arrives at the equation....At a pressure of 760 mm it computes a temperature of 4.5 °C.]"
- ^ Schumb, W. C.; Lynch, M. A. (1950). "Iodine Heptafluoride". Industrial & Engineering Chemistry. 42 (7): 1383–1386. doi:10.1021/ie50487a035.
- ^ Ruff & Keim 1930.
- ^ Beveridge, A. D.; Clark, H. C. (1967). "Pentahalides of the Transition Metals". In Gutmann, Viktor (ed.). Halogen Chemistry. Vol. 3. Academic Press. pp. 179–226. ISBN 9780323148474.
- ^ Кнунянц, И. Л. (1990). Химическая энциклопедия : в пяти томах (in Russian). Советская Энциклопедия. p. 496. ISBN 5-85270-008-8. OCLC 19556260.
Common sources edit
External links edit
- WebBook page for IF7
- National Pollutant Inventory - Fluoride and compounds fact sheet
- web elements listing