
KNOWPIA
WELCOME TO KNOWPIA
Summary
Iron(II) phosphate, also ferrous phosphate,[3] Fe3(PO4)2, is an iron salt of phosphoric acid.
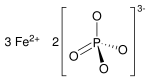
| |

| |
| Names | |
|---|---|
| IUPAC name
Iron(II) phosphate
| |
| Other names
Ferrous phosphate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider |
|
| ECHA InfoCard | 100.035.456 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Fe3(PO4)2 | |
| Appearance | brown powder |
| Density | 2.61 g/cm3 (octahydrate) |
| Melting point | 180 °C (356 °F; 453 K) (octahydrate) decomposes[1] |
| insoluble | |
| Structure | |
| monoclinic (octahydrate) | |
| C 2/m | |
a = 10.086 (octahydrate), b = 13.441 (octahydrate), c = 4.703 (octahydrate) α = 90°, β = 104.27°, γ = 90°
| |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P280, P304+P340, P305+P351+P338, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Natural occurrences edit
The mineral vivianite is a naturally occurring form of hydrated iron(II) phosphate.
Production edit
It can be formed by the reaction of ferrous hydroxide with phosphoric acid to produce hydrated iron(II) phosphate.
See also edit
References edit
External links edit
Media related to Iron(II) phosphate at Wikimedia Commons



