
KNOWPIA
WELCOME TO KNOWPIA
Summary
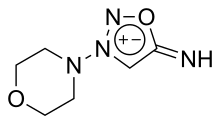
Linsidomine (3-morpholinosydnonimine or SIN-1[1]) is a vasodilator. It is a metabolite of the antianginal drug molsidomine and acts by releasing NO from the endothelial cells nonenzymatically. It also hyperpolarizes the cell membrane through influencing the sodium-potassium pump and thereby rendering it less responsive to adrenergic stimulation. Linsidomine injection at a dose of 1 mg produces usable erection[2] in about 70% of patients and full erection in up to 50% of patients. Linsidomine does not appear to be associated with priapism.[citation needed]
 | |
| Clinical data | |
|---|---|
| Other names | SIN-1 |
| AHFS/Drugs.com | International Drug Names |
| ATC code |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| Chemical and physical data | |
| Formula | C6H10N4O2 |
| Molar mass | 170.172 g·mol−1 |
| 3D model (JSmol) |
|
| |
| |
| (verify) | |
Linsidomine is neurotoxic and promotes oxidative stress on neurons.[3] Linsidomine is a peroxynitrite-generating compound involved in the pathogenesis of neurodegenerative diseases.[4]
References edit
- ^ Wen TC, Rogido MR, Moore JE, Genetta T, Peng H, Sola A (October 2005). "Cardiotrophin-1 protects cortical neuronal cells against free radical-induced injuries in vitro". Neuroscience Letters. 387 (1): 38–42. doi:10.1016/j.neulet.2005.07.018. PMID 16084018.
- ^ Lemaire A, Buvat J (June 1998). "[Erectile response to intracavernous injection of linsidomine in 38 impotent patients. Comparison with prostaglandin E1]". Progres en Urologie. 8 (3): 388–91. PMID 9689672.
- ^ Wallace DR, Dodson S, Nath A, Booze RM (January 2006). "Estrogen attenuates gp120- and tat1-72-induced oxidative stress and prevents loss of dopamine transporter function". Synapse. 59 (1): 51–60. doi:10.1002/syn.20214. PMID 16237680.
- ^ Jang JH, Aruoma OI, Jen LS, Chung HY, Surh YJ (February 2004). "Ergothioneine rescues PC12 cells from beta-amyloid-induced apoptotic death". Free Radical Biology & Medicine. 36 (3): 288–99. doi:10.1016/j.freeradbiomed.2003.11.005. PMID 15036348.


