
Summary
A lysophosphatidic acid (LPA) is a phospholipid derivative that can act as a signaling molecule.[1][2][3][4]
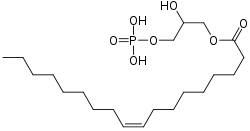
| |
| Names | |
|---|---|
| Systematic IUPAC name
(2R)-2-hydroxy-3-{[(9Z)-octadec-9-enoyl]oxy}propyl dihydrogen phosphate | |
| Other names
LPA
1-acyl-sn-glycerol 3-phosphate | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.040.631 |
| EC Number |
|
| |
| MeSH | lysophosphatidic+acid |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H41O7P | |
| Molar mass | 436.52 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Function edit
LPA acts as a potent mitogen due to its activation of three high-affinity G-protein-coupled receptors called LPAR1, LPAR2, and LPAR3 (also known as EDG2, EDG4, and EDG7). Additional, newly identified LPA receptors include LPAR4 (P2RY9, GPR23), LPAR5 (GPR92) and LPAR6 (P2RY5, GPR87).
Clinical significance edit
Because of its ability to stimulate cell proliferation, aberrant LPA-signaling has been linked to cancer in numerous ways. Dysregulation of autotaxin or the LPA receptors can lead to hyperproliferation, which may contribute to oncogenesis and metastasis.[5]
LPA may be the cause of pruritus (itching) in individuals with cholestatic (impaired bile flow) diseases.
GTPase activation edit
Downstream of LPA receptor activation, the small GTPase Rho can be activated, subsequently activating Rho kinase. This can lead to the formation of stress fibers and cell migration through the inhibition of myosin light-chain phosphatase.
Metabolism edit
There are a number of potential routes to its biosynthesis, but the most well-characterized is by the action of a lysophospholipase D called autotaxin, which removes the choline group from lysophosphatidylcholine.
Lysophosphatidic acids are also intermediates in the synthesis of phosphatidic acids.
See also edit
References edit
- ^ van Corven, Emile J.; Groenink, Alida; Jalink, Kees; Eichholtz, Thomas; Moolenaar, Wouter H. (1989-10-06). "Lysophosphatidate-induced cell proliferation: Identification and dissection of signaling pathways mediated by G proteins". Cell. 59 (1): 45–54. doi:10.1016/0092-8674(89)90868-4. PMID 2551506. S2CID 25154850.
- ^ Tsukahara, Tamotsu; Tsukahara, Ryoko; Haniu, Hisao; Matsuda, Yoshikazu; Murakami-Murofushi, Kimiko (2015-09-05). "Cyclic phosphatidic acid inhibits the secretion of vascular endothelial growth factor from diabetic human coronary artery endothelial cells through peroxisome proliferator-activated receptor gamma". Molecular and Cellular Endocrinology. 412: 320–329. doi:10.1016/j.mce.2015.05.021. hdl:10069/35888. ISSN 0303-7207. PMID 26007326. S2CID 10454566.
- ^ Moolenaar, Wouter H. (1995-06-02). "Lysophosphatidic Acid, a Multifunctional Phospholipid Messenger ∗". Journal of Biological Chemistry. 270 (22): 12949–12952. doi:10.1074/jbc.270.22.12949. ISSN 0021-9258. PMID 7768880.
- ^ Tigyi, Gabor; Parrill, Abby L. (2003-11-01). "Molecular mechanisms of lysophosphatidic acid action". Progress in Lipid Research. 42 (6): 498–526. doi:10.1016/S0163-7827(03)00035-3. ISSN 0163-7827. PMID 14559069.
- ^ Benesch, MG; Ko, YM; McMullen, TP; Brindley, DN (2014). "Autotaxin in the crosshairs: taking aim at cancer and other inflammatory conditions". FEBS Letters. 588 (16): 2712–27. doi:10.1016/j.febslet.2014.02.009. PMID 24560789. S2CID 35544825.
Further reading edit
- Kremer, Andreas E.; Martens, Job J.W.W.; Kulik, Wim; Ruëff, Franziska; Kuiper, Edith M.M.; Van Buuren, Henk R.; Van Erpecum, Karel J.; Kondrackiene, Jurate; et al. (2010). "Lysophosphatidic Acid is a Potential Mediator of Cholestatic Pruritus". Gastroenterology. 139 (3): 1008–18, 1018.e1. doi:10.1053/j.gastro.2010.05.009. PMID 20546739.
- Moolenaar, Wouter H. (1995). "Lysophosphatidic Acid, a Multifunctional Phospholipid Messenger". The Journal of Biological Chemistry. 270 (22): 12949–52. doi:10.1074/jbc.270.22.12949. PMID 7768880.
- Mills, Gordon B.; Moolenaar, Wouter H. (2003). "The emerging role of lysophosphatidic acid in cancer". Nature Reviews Cancer. 3 (8): 582–91. doi:10.1038/nrc1143. PMID 12894246. S2CID 29079135.
- Panupinthu, N; Lee, H Y; Mills, G B (2010). "Lysophosphatidic acid production and action: Critical new players in breast cancer initiation and progression". British Journal of Cancer. 102 (6): 941–6. doi:10.1038/sj.bjc.6605588. PMC 2844037. PMID 20234370.
- Park, S Y; Jeong, K J; Panupinthu, N; Yu, S; Lee, J; Han, J W; Kim, J M; Lee, J-S; et al. (2010). "Lysophosphatidic acid augments human hepatocellular carcinoma cell invasion through LPA1 receptor and MMP-9 expression". Oncogene. 30 (11): 1351–9. doi:10.1038/onc.2010.517. PMID 21102517.
- Yakubu, M A; Liliom, K; Tigyi, G J; Leffler, C W (1997). "Role of lysophosphatidic acid in endothelin-1-and hematoma-induced alteration of cerebral microcirculation". 273 (2): R703–R709. doi:10.1152/ajpregu.1997.273.2.R703.
{{cite journal}}: Cite journal requires|journal=(help) - Tigyi, G J; Hong, L; Yakubu, M; Parfenova, H; Shibata, M; Leffler, C W (1995). "Lysophosphatidic acid alters cerebrovascular reactivity in piglets". 268 (5): H2048–H2055. doi:10.1152/ajpheart.1995.268.5.H2048.
{{cite journal}}: Cite journal requires|journal=(help)


