
Summary
The manganese cycle is the biogeochemical cycle of manganese through the atmosphere, hydrosphere, biosphere and lithosphere. There are bacteria that oxidise manganese to insoluble oxides, and others that reduce it to Mn2+ in order to use it.[1]
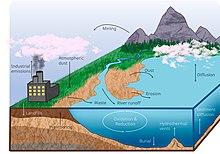
Manganese is a heavy metal that comprises about 0.1% of the Earth's crust and a necessary element for biological processes. It is cycled through the Earth in similar ways to iron, but with distinct redox pathways. Human activities have impacted the fluxes of manganese among the different spheres of the Earth.
Global manganese cycle edit
Manganese is a necessary element for biological functions such as photosynthesis, and some manganese oxidizing bacteria utilize this element in anoxic environments.[2][3] Movement of manganese (Mn) among the global "spheres" (described below) is mediated by both physical and biological processes. Manganese in the lithosphere enters the hydrosphere from erosion and dissolution of bedrock in rivers, in solution it then makes its way into the ocean. Once in the ocean, Mn can form minerals and sink to the ocean floor where the solid phase is buried. The global manganese cycle is being altered by anthropogenic influences, such as mining and mineral processing for industrial use, as well as through the burning of fossil fuels.[4]
Lithosphere edit
Manganese is the tenth most abundant metal in the Earth's crust, making up approximately 0.1% of the total composition, or about 0.019 mol kg−1, which is found mostly in the oceanic crust.[5] [6]
Crust edit
Manganese (Mn) commonly precipitates in igneous rocks in the form of early-stage crystalline minerals, which, once exposed to water and/or oxygen, are highly soluble and easily oxidized to form Mn oxides on the surfaces of rocks.[7] Dendritic crystals rich in Mn form when microbes reprecipitate the Mn from the rocks on which they develop onto the surface after utilizing the Mn for their metabolism. For certain cyanobacteria found on desert varnish samples, for example, it has been found that manganese is used as a catalytic antioxidant to facilitate survival in the harsh sunlight and water conditions they face on desert rock surfaces.[8]
Soil edit
Manganese is an important soil micronutrient for plant growth, playing an essential role as a catalyst in the oxygen-evolving complex of photosystem II, a photosynthetic pathway. [6] Soil fungi in particular have been found to oxidize the reduced, soluble form of manganese (Mn2+) under anaerobic conditions, and may reprecipitate it as manganese oxides (Mn+3 to Mn+7) under aerobic conditions, where the preferred metabolic pathway typically involves the utilization of oxygen. [9][10] Although not all iron-reducing bacteria have the capability of reducing manganese, there is overlap in the taxa that can perform both metabolisms; these organisms are very common in a range of environmental conditions. Challenges however persist in isolating these microbes in cultures.[11][12]
Depending on the pH, organic substrate availability, and oxygen concentration, Mn can either behave as an oxidation catalyst or an electron receptor. [13] Though much of the total Mn that is cycled in soil is biologically-mediated, some inorganic reactions also contribute to Mn oxidation or precipitation of Mn oxides. The reduction potential (pe) and pH are two known constraints on the solubility of Mn in soils. [13] As pH increases, Mn speciation becomes less sensitive to variations in pe. In acidic (pH = 5) soils with high reduction potentials (pe > 8), the forms of Mn are mostly reducible, with exchangeable and soluble Mn decreasing dramatically in concentration with increases in pe. [13] Mn is also found in inorganic chelation complexes, where Mn forms coordinate bonds with SO42-, HCO3−, and Cl− ions. These complexes are important for organic matter stabilization in soils, as they have high surface areas and interact with organic matter through adsorption. [14]
Hydrosphere edit
Iron (Fe) and Manganese (Mn) similarities in their respective cycles and are often studied together. Both have similar sources in the hydrosphere, which are hydrothermal vent fluxes,[16] dust inputs,[17] and weathering of rocks.[18][19] The major removal of Mn from the ocean involves similar processes to Fe as well, with the most abundant removal from the hydrosphere via biological uptake,[20] oxidative precipitation,[21] and scavenging.[22] Microorganisms oxidize the bioavailable Mn(II) to form Mn(IV), an insoluble manganese oxide that aggregates to form particulate matter that can then sink to the ocean floor.[23] Manganese is important in aquatic ecosystems for photosynthesis and other biological functions.[2]
Freshwater and estuary edit
Advection from tidal flows re-suspends estuary beds and can unearth manganese.[24] The particulate manganese is dissolved via reduction that forms Mn (II), adding it to the internal cycle of manganese in organisms in the ecosystem. Estuary biogeochemistry is heavily influenced by tidal oscillations, temperature, and pH changes, and thus the manganese input into the internal cycling is variable.[24] Mn in rivers and streams typically has a lower residence time than estuaries, and a large majority of the Mn is soluble Mn (II).[25] In these freshwater ecosystems, the manganese cycling is depended on sediment fluxes that provide an influx of Mn into the system. Oxidation of Mn (II) from sediment drives the redox reactions that fuel the biogeochemical processes with Mn, as well as Mn reducing microbes.[26]
Marine edit
In the ocean, different patterns of manganese cycling are seen. In the photic zone, there is a decrease in Mn particulate formation during the daytime, as rates of microbially catalyzed oxidation decrease and photo-dissolution of Mn oxides increases.[26] The GEOTRACES program has led the production of the first global manganese model, in which predictions of global manganese distribution can be made.[27] This global model found strong removal rates of Mn as water moves from the Atlantic Ocean surface to the North Atlantic deep water resulting in Mn depletion in water moving southward along the thermohaline conveyor.[26] Overall, when looking at organism interactions with manganese, it is known that redox reactions play a key role, as well as that Mn has important biological functions, however far less is known about uptake and remineralization processes such as with iron.[26]
Early Earth edit
Terrestrial manganese has existed since the formation of Earth around 4.6 Ga.[28] The Sun and the Solar System formed during the collapse of a molecular cloud populated with many trace metals, including manganese.[29] The chemical composition of the molecular cloud determined the composition of the many celestial bodies that form within it.[29] Nearby supernova explosions populated the cloud with manganese; the most common manganese-forming supernovae are Type Ia supernovae.[29][30]
The early Earth contained very little free oxygen (O2) until the Great Oxygenation Event around 2.35 Ga.[31][32][33][34][35] Without O2, redox cycling of Mn was limited. Instead, soluble Mn(II) was only released into the oceans via silicate weathering on igneous rocks and supplied through hydrothermal vents.[36] The increase in Mn oxidation occurred during the Archean Eon (> 2.5 Ga), whereas the first evidence of manganese redox cycling appears ~ 2.4 Ga, before the Great Oxygenation Event and during the Paleoproterozoic Era.[36][37]
Although the Great Oxygenation Event raised the abundance of oxygen on Earth, the oxygen levels were still relatively low compared to modern levels.[38] It is believed that many primary producers were anoxygenic phototrophs and took advantage of abundant hydrogen sulfide (H2S) to catalyze photosynthesis.[39][40][41] Anoxygenic phototrophy and oxygenic photosynthesis both require electron donors, with all known forms of anoxygenic phototrophy relying on reaction center electron acceptors with reduction potentials around 250-500 mV. Oxygenic photosynthesis requires reduction potentials around 1250 mV.[36] It has been hypothesized that this wide difference in reduction potential indicates an evolutionary missing link in the origin of oxygenic photosynthesis.[36] Mn(II) is the leading candidate for bridging this gap.[40][42] The water-oxidizing complex, a key component of PSII, begins with the oxidation of Mn(II), which, along with additional evidence, strongly supports the hypothesis that manganese was a necessary step in the evolution of oxygenic photosynthesis.[36][37][40][42][43]
Anthropogenic influences edit
While manganese naturally occurs in the environment, the global Mn cycle is influenced through anthropogenic activities. Mn is utilized in many commercial products, such as fireworks, leather, paint, glass, fertilizer, animal feed, and dry cell batteries.[44] However, the effect of Mn pollution from these sources is minor compared to that of mining and mineral processing.[4] The burning of fossil fuels, such as coal and natural gas, further contribute to the anthropogenic cycling of Mn.[45]
Mining and mineral processing edit
Anthropogenic influences on the manganese cycle mainly stem from industrial mining and mineral processing, specifically, within the iron and steel industries.[4] Mn is used in iron and steel production to improve hardness, strength, and stiffness,[4] and is the primary component used in low-cost stainless steel and aluminum alloy production.[46] Anthropogenic mining and mineral processing has spread Mn through three methods: wastewater discharge, industrial emissions, and releases in soils.[47]
Wastewater discharge edit
Waste from mining and mineral processing facilities is typically separated into liquid and solid forms.[48] Due to insufficient management and poor mining processes, especially in developing countries, liquid waste containing Mn can be discharged into bodies of water through anthropogenic effluents.[49] Domestic wastewater and sewage sludge disposal are the main anthropogenic sources of Mn within aquatic ecosystems.[48] In marine systems, the disposal of mine tailings contributes to aquatic anthropogenic Mn concentrations[50] where high levels can be toxic to marine life.[51]
Industrial emissions edit
The main anthropogenic influence of Mn input to the atmosphere is through industrial emissions,[52] and roughly 80% of industrial emissions of Mn is due to steel and iron processing facilities.[53] In the Northern Hemisphere, some of the Mn pollutants released through industrial emissions are transferred to Arctic regions through atmospheric circulation, where particulates settle and accumulate in natural bodies of water.[54][55]
Such atmospheric pollution of Mn can be hazardous for humans working or living near industrial facilities. Dust and smoke containing manganese dioxide and manganese tetroxide released into the air during mining is a primary cause of manganism in humans.[56]
Releases in soils edit
The solid waste disposal of substances containing Mn by industrial sources typically ends up in landfills.[4] Additional Mn deposition in soils can result from particulate settling of Mn released through industrial emissions.[4] An analysis of datasets on the soil chemistry of North America and Europe revealed greater than 50% of Mn in ridge soils near iron or steel processing facilities was attributed to anthropogenic industrial inputs, whether through solid waste disposal or previously airborne particulates depositing in soils.[57]
Burning of fossil fuels edit
Anthropogenically sourced Mn from the burning of fossil fuels has been found in the atmosphere, hydrosphere, and lithosphere.[45] Mn is a trace element in fly ash, a residue from the use of coal for power production, which often ends up in the atmosphere, soils, and bodies of water.[58] Methylcyclopentadienyl Mn tricarbonyl (MMT), a gasoline additive containing Mn, also contributes to Mn anthropogenic cycling.[59] Due to the use of MMT as a fuel additive, motor vehicles are a significant source of Mn in the atmosphere, especially in regions of high traffic activity.[60] In some regions, roughly 40% of Mn in the atmosphere was due to exhaust from traffic.[60] Particulate manganese phosphate, manganese sulfate, and manganese oxide are the primary emissions from MMT combustion through its usage in gasoline.[61][62] A portion of these particulates eventually leave the atmosphere to settle in soils and bodies of water.[45]
References edit
- ^ Ehrlich, Henry Lutz; Newman, Dianne K. (22 December 2008). Geomicrobiology. CRC Press. pp. 347–426. ISBN 978-0-8493-7907-9.
- ^ a b Raven, John A. (1990-09-01). "Predictions of Mn and Fe use efficiencies of phototrophic growth as a function of light availability for growth and of C assimilation pathway". New Phytologist. 116 (1): 1–18. doi:10.1111/j.1469-8137.1990.tb00505.x. ISSN 0028-646X.
- ^ Wang, Xuan; Xie, Guo-Jun; Tian, Ning; Dang, Cheng-Cheng; Cai, Chen; Ding, Jie; Liu, Bing-Feng; Xing, De-Feng; Ren, Nan-Qi; Wang, Qilin (2022-05-20). "Anaerobic microbial manganese oxidation and reduction: A critical review". Science of the Total Environment. 822: 153513. doi:10.1016/j.scitotenv.2022.153513. ISSN 0048-9697. PMID 35101498. S2CID 246434330.
- ^ a b c d e f Howe, Paul; Malcolm, Heath; WHO; Dobson, Stuart; Organization, World Health (2004-12-17). Manganese and Its Compounds: Environmental Aspects. World Health Organization. ISBN 978-92-4-153063-7.
- ^ Post, Jeffrey E. (1999-03-30). "Manganese oxide minerals: Crystal structures and economic and environmental significance". Proceedings of the National Academy of Sciences. 96 (7): 3447–3454. doi:10.1073/pnas.96.7.3447. ISSN 0027-8424. PMC 34287. PMID 10097056.
- ^ a b Schmidt, Sidsel Birkelund; Jensen, Poul Erik; Husted, Søren (2016-07-01). "Manganese Deficiency in Plants: The Impact on Photosystem II". Trends in Plant Science. 21 (7): 622–632. doi:10.1016/j.tplants.2016.03.001. ISSN 1360-1385. PMID 27150384.
- ^ Post, Jeffrey E. (1999-03-30). "Manganese oxide minerals: Crystal structures and economic and environmental significance". Proceedings of the National Academy of Sciences. 96 (7): 3447–3454. doi:10.1073/pnas.96.7.3447. ISSN 0027-8424. PMC 34287. PMID 10097056.
- ^ Lingappa, Usha F.; Yeager, Chris M.; Sharma, Ajay; Lanza, Nina L.; Morales, Demosthenes P.; Xie, Gary; Atencio, Ashley D.; Chadwick, Grayson L.; Monteverde, Danielle R.; Magyar, John S.; Webb, Samuel M.; Valentine, Joan Selverstone; Hoffman, Brian M.; Fischer, Woodward W. (2021-06-22). "An ecophysiological explanation for manganese enrichment in rock varnish". Proceedings of the National Academy of Sciences. 118 (25): e2025188118. doi:10.1073/pnas.2025188118. ISSN 0027-8424. PMC 8237629. PMID 34161271.
- ^ Mann, P. J. G.; Quastel, J. H. (August 1946). "Manganese Metabolism in Soils". Nature. 158 (4005): 154–156. doi:10.1038/158154a0. ISSN 1476-4687. S2CID 43335147.
- ^ Thompson, Ian A.; Huber, Don M.; Guest, Chris A.; Schulze, Darrell G. (2005-06-25). "Fungal manganese oxidation in a reduced soil". Environmental Microbiology. 7 (9): 1480–1487. doi:10.1111/j.1462-2920.2005.00842.x. ISSN 1462-2912. PMID 16104870.
- ^ Ghiorse, W. C. (1984-10-01). "Biology of Iron-And Manganese-Depositing Bacteria". Annual Review of Microbiology. 38 (1): 515–550. doi:10.1146/annurev.mi.38.100184.002503. ISSN 0066-4227. PMID 6388499.
- ^ Ghiorse, William C. (1988), Graham, Robin D.; Hannam, Robert J.; Uren, Nicholas C. (eds.), "The Biology of Manganese Transforming Microorganisms in Soil", Manganese in Soils and Plants: Proceedings of the International Symposium on ‘Manganese in Soils and Plants’ held at the Waite Agricultural Research Institute, The University of Adelaide, Glen Osmond, South Australia, August 22–26, 1988 as an Australian Bicentennial Event, Dordrecht: Springer Netherlands, pp. 75–85, doi:10.1007/978-94-009-2817-6_6, ISBN 978-94-009-2817-6, retrieved 2022-10-17
- ^ a b c Norvell, W. A. (1988), Graham, Robin D.; Hannam, Robert J.; Uren, Nicholas C. (eds.), "Inorganic Reactions of Manganese in Soils", Manganese in Soils and Plants: Proceedings of the International Symposium on ‘Manganese in Soils and Plants’ held at the Waite Agricultural Research Institute, The University of Adelaide, Glen Osmond, South Australia, August 22–26, 1988 as an Australian Bicentennial Event, Dordrecht: Springer Netherlands, pp. 37–58, doi:10.1007/978-94-009-2817-6_4, ISBN 978-94-009-2817-6, retrieved 2022-10-17
- ^ Grygo-Szymanko, Emilia; Tobiasz, Anna; Walas, Stanisław (2016-06-01). "Speciation analysis and fractionation of manganese: A review". TrAC Trends in Analytical Chemistry. 80: 112–124. doi:10.1016/j.trac.2015.09.010. ISSN 0165-9936.
- ^ van Hulten, Marco; Dutay, Jean-Claude; Middag, Rob; de Baar, Hein; Roy-Barman, Matthieu; Gehlen, Marion; Tagliabue, Alessandro; Sterl, Andreas (2016-07-11). "Manganese in the world ocean: a first global model" (PDF). doi:10.5194/bg-2016-282.
{{cite journal}}: Cite journal requires|journal=(help) - ^ German, C. R.; Casciotti, K. A.; Dutay, J.-C.; Heimbürger, L. E.; Jenkins, W. J.; Measures, C. I.; Mills, R. A.; Obata, H.; Schlitzer, R.; Tagliabue, A.; Turner, D. R.; Whitby, H. (2016-11-28). "Hydrothermal impacts on trace element and isotope ocean biogeochemistry". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 374 (2081): 20160035. doi:10.1098/rsta.2016.0035. PMC 5069535. PMID 29035265.
- ^ Arimoto, R.; Duce, R. A.; Ray, B. J.; Unni, C. K. (1985). "Atmospheric trace elements at Enewetak Atoll: 2. Transport to the ocean by wet and dry deposition". Journal of Geophysical Research. 90 (D1): 2391. doi:10.1029/JD090iD01p02391. ISSN 0148-0227.
- ^ Froelich, P. N.; Klinkhammer, G. P.; Bender, M. L.; Luedtke, N. A.; Heath, G. R.; Cullen, Doug; Dauphin, Paul; Hammond, Doug; Hartman, Blayne; Maynard, Val (1979-07-01). "Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic: suboxic diagenesis". Geochimica et Cosmochimica Acta. 43 (7): 1075–1090. doi:10.1016/0016-7037(79)90095-4. ISSN 0016-7037.
- ^ Jensen, Laramie T.; Morton, Peter; Twining, Benjamin S.; Heller, Maija I.; Hatta, Mariko; Measures, Christopher I.; John, Seth; Zhang, Ruifeng; Pinedo-Gonzalez, Paulina; Sherrell, Robert M.; Fitzsimmons, Jessica N. (2020-11-01). "A comparison of marine Fe and Mn cycling: U.S. GEOTRACES GN01 Western Arctic case study". Geochimica et Cosmochimica Acta. 288: 138–160. doi:10.1016/j.gca.2020.08.006. ISSN 0016-7037. S2CID 224924072.
- ^ Sunda, William (2012). "Feedback Interactions between Trace Metal Nutrients and Phytoplankton in the Ocean". Frontiers in Microbiology. 3: 204. doi:10.3389/fmicb.2012.00204. ISSN 1664-302X. PMC 3369199. PMID 22701115.
- ^ Bruland, K.W.; Lohan, M.C. (2003), "Controls of Trace Metals in Seawater", Treatise on Geochemistry, Elsevier, pp. 23–47, doi:10.1016/b0-08-043751-6/06105-3, ISBN 9780080437514, retrieved 2022-10-28
- ^ Balistrieri, L.; Brewer, P.G.; Murray, J.W. (1981-02-01) [1981-02]. "Scavenging residence times of trace metals and surface chemistry of sinking particles in the deep ocean". Deep Sea Research Part A. Oceanographic Research Papers. 28 (2): 101–121. doi:10.1016/0198-0149(81)90085-6. ISSN 0198-0149.
- ^ van Hulten, Marco; Dutay, Jean-Claude; Middag, Rob; de Baar, Hein; Roy-Barman, Matthieu; Gehlen, Marion; Tagliabue, Alessandro; Sterl, Andreas (2016-07-11). "Manganese in the world ocean: a first global model" (PDF). doi:10.5194/bg-2016-282.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b Morris, A. W.; Bale, A. J.; Howland, R. J. M. (1982-02-01). "The dynamics of estuarine manganese cycling". Estuarine, Coastal and Shelf Science. 14 (2): 175–192. doi:10.1016/S0302-3524(82)80044-3. ISSN 0272-7714.
- ^ Laxen, Duncan P. H.; Davison, William; Woof, Colin (1984-10-01). "Manganese chemistry in rivers and streams". Geochimica et Cosmochimica Acta. 48 (10): 2107–2111. doi:10.1016/0016-7037(84)90390-9. ISSN 0016-7037.
- ^ a b c d Buffle, Jacques; Leeuwen, Herman P. van (2018-10-24). Revival: Environmental Particles (1993): Volume 2. CRC Press. ISBN 978-1-351-27079-3.
- ^ van Hulten, Marco; Dutay, Jean-Claude; Middag, Rob; de Baar, Hein; Roy-Barman, Matthieu; Gehlen, Marion; Tagliabue, Alessandro; Sterl, Andreas (2016-07-11). "Manganese in the world ocean: a first global model" (PDF). doi:10.5194/bg-2016-282.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Bouvier, Audrey; Wadhwa, Meenakshi (2010-08-22). "The age of the Solar System redefined by the oldest Pb–Pb age of a meteoritic inclusion". Nature Geoscience. 3 (9): 637–641. doi:10.1038/ngeo941. ISSN 1752-0908.
- ^ a b c Carroll, Bradley W.; Ostlie, Dale A. (2017-10-29). An Introduction to Modern Astrophysics (2 ed.). Cambridge University Press. doi:10.1017/9781108380980. ISBN 978-1-108-42216-1.
- ^ Leung, Shing-Chi; Nomoto, Ken’ichi (2018-07-13). "Explosive Nucleosynthesis in Near-Chandrasekhar-mass White Dwarf Models for Type Ia Supernovae: Dependence on Model Parameters". The Astrophysical Journal. 861 (2): 143. arXiv:1710.04254. doi:10.3847/1538-4357/aac2df. ISSN 1538-4357. S2CID 250859408.
- ^ Fischer, Woodward W; Hemp, James; Valentine, Joan Selverstone (2016-04-01). "How did life survive Earth's great oxygenation?". Current Opinion in Chemical Biology. Biocatalysis and biotransformation * Bioinorganic chemistry. 31: 166–178. doi:10.1016/j.cbpa.2016.03.013. ISSN 1367-5931. PMID 27043270.
- ^ Johnson, Jena E.; Gerpheide, Aya; Lamb, Michael P.; Fischer, Woodward W. (2014-05-01). "O 2 constraints from Paleoproterozoic detrital pyrite and uraninite". Geological Society of America Bulletin. 126 (5–6): 813–830. doi:10.1130/B30949.1. ISSN 0016-7606.
- ^ Farquhar, James; Bao, Huiming; Thiemens, Mark (2000-08-04). "Atmospheric Influence of Earth's Earliest Sulfur Cycle". Science. 289 (5480): 756–758. doi:10.1126/science.289.5480.756. ISSN 0036-8075. PMID 10926533.
- ^ Paris, G.; Adkins, J. F.; Sessions, A. L.; Webb, S. M.; Fischer, W. W. (2014-11-07). "Neoarchean carbonate–associated sulfate records positive Δ 33 S anomalies". Science. 346 (6210): 739–741. doi:10.1126/science.1258211. ISSN 0036-8075. PMID 25378622. S2CID 20532947.
- ^ Torres, Mark A.; Paris, Guillaume; Adkins, Jess F.; Fischer, Woodward W. (2018-09-23). "Riverine evidence for isotopic mass balance in the Earth's early sulfur cycle". Nature Geoscience. 11 (9): 661–664. doi:10.1038/s41561-018-0184-7. hdl:1911/103259. ISSN 1752-0894. S2CID 133714335.
- ^ a b c d e Lingappa, Usha F.; Monteverde, Danielle R.; Magyar, John S.; Valentine, Joan Selverstone; Fischer, Woodward W. (2019-08-20). "How manganese empowered life with dioxygen (and vice versa)". Free Radical Biology and Medicine. Early Life on Earth and Oxidative Stress. 140: 113–125. doi:10.1016/j.freeradbiomed.2019.01.036. ISSN 0891-5849. PMID 30738765. S2CID 73436722.
- ^ a b Johnson, Jena E.; Webb, Samuel M.; Thomas, Katherine; Ono, Shuhei; Kirschvink, Joseph L.; Fischer, Woodward W. (2013-07-09). "Manganese-oxidizing photosynthesis before the rise of cyanobacteria". Proceedings of the National Academy of Sciences. 110 (28): 11238–11243. doi:10.1073/pnas.1305530110. ISSN 0027-8424. PMC 3710856. PMID 23798417.
- ^ Lyons, Timothy W.; Reinhard, Christopher T.; Planavsky, Noah J. (2014-02-19). "The rise of oxygen in Earth's early ocean and atmosphere". Nature. 506 (7488): 307–315. doi:10.1038/nature13068. ISSN 1476-4687. PMID 24553238. S2CID 4443958.
- ^ Olson, Kenneth R.; Straub, Karl D. (2016-01-01). "The Role of Hydrogen Sulfide in Evolution and the Evolution of Hydrogen Sulfide in Metabolism and Signaling". Physiology. 31 (1): 60–72. doi:10.1152/physiol.00024.2015. ISSN 1548-9213. PMID 26674552.
- ^ a b c Fischer, Woodward W.; Hemp, James; Johnson, Jena E. (2016-06-29). "Evolution of Oxygenic Photosynthesis". Annual Review of Earth and Planetary Sciences. 44 (1): 647–683. doi:10.1146/annurev-earth-060313-054810. ISSN 0084-6597.
- ^ Tice, Michael M.; Lowe, Donald R. (2004-09-30). "Photosynthetic microbial mats in the 3,416-Myr-old ocean". Nature. 431 (7008): 549–552. doi:10.1038/nature02888. ISSN 0028-0836. PMID 15457255. S2CID 4412591.
- ^ a b Fischer, Woodward W.; Hemp, James; Johnson, Jena E. (2015-05-29). "Manganese and the Evolution of Photosynthesis". Origins of Life and Evolution of Biospheres. 45 (3): 351–357. doi:10.1007/s11084-015-9442-5. ISSN 0169-6149. PMID 26017176. S2CID 254894651.
- ^ Hemp, James; Lücker, Sebastian; Schott, Joachim; Pace, Laura A; Johnson, Jena E; Schink, Bernhard; Daims, Holger; Fischer, Woodward W (2016-04-19). "Genomics of a phototrophic nitrite oxidizer: insights into the evolution of photosynthesis and nitrification". The ISME Journal. 10 (11): 2669–2678. doi:10.1038/ismej.2016.56. ISSN 1751-7362. PMC 5113846. PMID 27093047.
- ^ Chakraborty, Sudipta; Martinez-Finley, Ebany; Caito, Sam; Chen, Pan; Aschner, Michael (2014-07-09), "CHAPTER 9:Manganese", Binding, Transport and Storage of Metal Ions in Biological Cells, Metallobiology, pp. 260–281, doi:10.1039/9781849739979-00260, ISBN 978-1-84973-599-5, retrieved 2022-11-09
- ^ a b c Williams, Malcolm; Todd, G Daniel; Roney, Nickolette; Crawford, Jewell; Coles, Charleton; McClure, Peter R; Garey, Joan D; Zaccaria, Kimberly; Citra, Mario (2012). "Toxicological Profile for Manganes". Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles. Agency for Toxic Substances and Disease Registry (US). PMID 24049862.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Tangstad, Merete (2013-01-01), Gasik, Michael (ed.), "Chapter 7 - Manganese Ferroalloys Technology", Handbook of Ferroalloys, Oxford: Butterworth-Heinemann, pp. 221–266, ISBN 978-0-08-097753-9, retrieved 2022-11-16
- ^ Nkele, K.; Mpenyana-Monyatsi, L.; Masindi, V. (2022-12-01). "Challenges, advances and sustainabilities on the removal and recovery of manganese from wastewater: A review". Journal of Cleaner Production. 377: 134152. doi:10.1016/j.jclepro.2022.134152. ISSN 0959-6526. S2CID 252482228.
- ^ a b Nriagu, Jerome O.; Pacyna, Jozef M. (1988). "Quantitative assessment of worldwide contamination of air, water and soils by trace metals". Nature. 333 (6169): 134–139. doi:10.1038/333134a0. ISSN 1476-4687. PMID 3285219. S2CID 4262601.
- ^ Nkele, K.; Mpenyana-Monyatsi, L.; Masindi, V. (2022-12-01). "Challenges, advances and sustainabilities on the removal and recovery of manganese from wastewater: A review". Journal of Cleaner Production. 377: 134152. doi:10.1016/j.jclepro.2022.134152. ISSN 0959-6526. S2CID 252482228.
- ^ Florence, T. M.; Stauber, J. L.; Ahsanullah, M. (1994-06-06). "Toxicity of nickel ores to marine organisms". Science of the Total Environment. Nickel Biochemistry, Toxicology, and Ecologic Issues. 148 (2): 139–155. doi:10.1016/0048-9697(94)90391-3. ISSN 0048-9697. PMID 8029690.
- ^ Marks, Becky; Peters, Adam; McGough, Doreen (2017-01-01). "Aquatic environmental risk assessment of manganese processing industries". NeuroToxicology. 58: 187–193. doi:10.1016/j.neuro.2016.04.011. ISSN 0161-813X. PMID 27090824. S2CID 24866936.
- ^ Lioy, P. J. (1983). "Air pollution emission profiles of toxic and trace elements from energy related sources: status and needs". Neurotoxicology. 4 (3): 103–112. ISSN 0161-813X. PMID 6686299.
- ^ "Manganese: Potential for Exposure" (PDF).
- ^ Varotsos, Costas A.; Krapivin, Vladimir F. (2018-10-25). "Pollution of Arctic Waters Has Reached a Critical Point: an Innovative Approach to This Problem". Water, Air, & Soil Pollution. 229 (11): 343. doi:10.1007/s11270-018-4004-x. ISSN 1573-2932. S2CID 105926567.
- ^ Danilov, Aleksandr Sergeevich; Smirnov, Yuri Dmitrievich; Pashkevich, Maria Anatolievna (2015-11-03). "Use of Biological Adhesive for Effective Dust Suppression in Mining Operations". Journal of Ecological Engineering. 16 (5): 9–14. doi:10.12911/22998993/60448. ISSN 2299-8993.
- ^ Schiele, R (1991). Metals and their compounds in the environment - occurrence, analysis, and biological relevance. Weinheim VCH. pp. 1035–1044.
- ^ Herndon, Elizabeth M.; Jin, Lixin; Brantley, Susan L. (2011-01-01). "Soils Reveal Widespread Manganese Enrichment from Industrial Inputs". Environmental Science & Technology. 45 (1): 241–247. doi:10.1021/es102001w. ISSN 0013-936X. PMID 21133425.
- ^ Ibrahim, lubna Abdel Aziz (2015-10-01). "Chemical characterization and mobility of metal species in fly ash–water system". Water Science. 29 (2): 109–122. doi:10.1016/j.wsj.2015.10.001. ISSN 1110-4929. S2CID 101064919.
- ^ Chen, Pan; Culbreth, Megan; Aschner, Michael (2016-07-01). "Exposure, epidemiology, and mechanism of the environmental toxicant manganese". Environmental Science and Pollution Research. 23 (14): 13802–13810. doi:10.1007/s11356-016-6687-0. ISSN 1614-7499. PMID 27102617. S2CID 34209604.
- ^ a b Davis, D. W.; Hsiao, K.; Ingels, R.; Shikiya, J. (1988). "Origins of manganese in air particulates in California". JAPCA. 38 (9): 1152–1157. doi:10.1080/08940630.1988.10466464. ISSN 0894-0630. PMID 3230404.
- ^ Ter Haar, G. L.; Grifffing, M. E.; Brandt, M.; Oberding, D. G.; Kapron, M. (1975-08-01). "Methylcyclopentadienyl Manganese Tricarbonyl as an Antiknock: Composition and Fate of Manganese Exhaust Products". Journal of the Air Pollution Control Association. 25 (8): 858–859. doi:10.1080/00022470.1975.10470152. ISSN 0002-2470.
- ^ Lynam, D R; Roos, J W; Pfeifer, G D; Fort, B F; Pullin, T G (1999-04-01). "Environmental effects and exposures to manganese from use of methylcyclopentadienyl manganese tricarbonyl (MMT) in gasoline". Neurotoxicology. 20 (2–3): 145–150. ISSN 1872-9711. PMID 10385878.


