
Summary
Methyl aminolevulinate (MAL) is a drug used as a sensitizer in photodynamic therapy. It is a prodrug that is metabolized to protoporphyrin IX. It is marketed as Metvix.
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Multum Consumer Information |
| ATC code |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) |
|
| Chemical and physical data | |
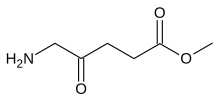
| Formula | C6H11NO3 |
| Molar mass | 145.158 g·mol−1 |
| 3D model (JSmol) |
|
| |
| |
| | |
Metvix cream is applied topically and some time later the skin is illuminated with a proprietary red light (630 nm) source (medical lamp 'Aktilite') to activate the photosensitiser.
Metvix is developed by Photocure and Galderma has bought all rights to Metvix.[1]
Approvals and indications edit
Methyl aminolevulinate is approved in New Zealand for treatment of basal cell carcinoma.[2]
It is now approved in many countries and has been used to treat non-melanoma skin cancer (including basal cell carcinoma).[3]
It has some advantages over Levulan.[4]
It has been reported as controversial in some quarters, with severe pain allegedly being experienced by some patients. [5]
References edit
- ^ "Photocure Divests Metvix to Galderma for EUR 51 Million". Archived from the original on 2011-02-07. Retrieved 2010-09-10.
- ^ Ngan V (2003). "Methyl aminolevulinate photodynamic therapy (MAL PDT)". DermNet NZ.
- ^ "New 5 Year Metvix-PDT Data Demonstrate Long-Term Efficacy & Reliability For NM Skin Cancer Treatment". EurekAlert!. 2006.
- ^ O'Connor AE, Gallagher WM, Byrne AT (2009). "Porphyrin and nonporphyrin photosensitizers in oncology: preclinical and clinical advances in photodynamic therapy". Photochemistry and Photobiology. 85 (5): 1053–74. doi:10.1111/j.1751-1097.2009.00585.x. PMID 19682322. S2CID 205950773.
- ^ "Concerns raised over ALA skin cancer cream as patients recount 'horrendous' pain". Australian Broadcasting Corporation. 16 November 2013.


