
Summary
Pyrithione is the common name of an organosulfur compound with molecular formula C
5H
5NOS, chosen as an abbreviation of pyridinethione, and found in the Persian shallot.[4] It exists as a pair of tautomers, the major form being the thione 1-hydroxy-2(1H)-pyridinethione and the minor form being the thiol 2-mercaptopyridine N-oxide; it crystallises in the thione form.[5] It is usually prepared from either 2-bromopyridine,[1] 2-chloropyridine,[6][7] or 2-chloropyridine N-oxide,[8] and is commercially available as both the neutral compound and its sodium salt.[1] It is used to prepare zinc pyrithione,[9][10] which is used primarily to treat dandruff and seborrhoeic dermatitis in medicated shampoos,[11][12] though is also an anti-fouling agent in paints.[13]
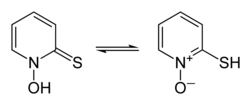 Interconversion of pyrithione tautomers
thione form on the left, thiol form on the right | |
| Names | |
|---|---|
| Preferred IUPAC name
1-Hydroxy-2(1H)-pyridinethione (thione) 2-Pyridinethiol 1-oxide (thiol) | |
| Other names
Omadine
thione: 1-Hydroxypyridine-2-thione N-Hydroxypyridine-2-thione thiol: 2-Mercaptopyridine monoxide 2-Mercaptopyridine N-oxide 2-Mercaptopyridine 1-oxide | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| 109936 | |
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| DrugBank |
|
| ECHA InfoCard | 100.013.027 |
| EC Number |
|
| 913415 | |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H5NOS | |
| Molar mass | 127.16 g·mol−1 |
| Appearance | Beige crystalline powder |
| Melting point | 70 to 73 °C (158 to 163 °F; 343 to 346 K) |
| 2.5 g L−1 at 20 °C | |
| Solubility | Soluble: benzene, chloroform, dichloromethane, dimethylformamide, dimethylsulfoxide, ethyl acetate[1] Slightly soluble: diethyl ether, ethanol, methyl tert-butyl ether, tetrahydrofuran[1] |
| Acidity (pKa) | −1.95 (proton addition), 4.6[2][3] |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H301, H315, H319, H335 | |
| P261, P264, P270, P271, P280, P301+P310, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Preparation edit
The preparation of pyrithione was first reported in 1950[13] by Shaw[14] and was prepared by reaction of 2-chloropyridine N-oxide with sodium hydrosulfide followed by acidification,[8] or more recently with sodium sulfide.[15] 2-chloropyridine N-oxide itself can be prepared from 2-chloropyridine using peracetic acid.[16] Another approach involves treating the same starting N-oxide with thiourea to afford pyridyl-2-isothiouronium chloride N-oxide which undergoes base hydrolysis to pyrithione.[1][17] 2-Bromopyridine can be oxidised to its N-oxide using a suitable peracid (as per 2-chloropyridine), both approaches being analogous to that reported in Organic Syntheses for the oxidation of pyridine to its N-oxide.[1][18] A substitution reaction using either sodium dithionite (Na
2S
2O
4) or sodium sulfide with sodium hydroxide will allow the replacement of the bromo substituent with a thiol functional group.[1][15]
The alternative strategy is to form the mercaptan before introducing the N-oxide moiety. 2-Mercaptopyridine was originally synthesized in 1931 by heating 2-chloropyridine with calcium hydrosulfide,[6] an approach similar that first used to prepare pyrithione.[8] The analogous thiourea approach via a uronium salt was reported in 1958 and provides a more convenient route to 2-mercaptopyridine.[7] Oxidation to the N-oxide can then be undertaken.
Pyrithione is found as a natural product in the Allium stipitatum plant, an Asian species of onion, also known as the Persian shallot.[4] Its presence was detected using positive ion mass spectrometry using a DART ion source[19] and the disulfide dipyrithione (2,2'-disulfanediylbis(pyridine)-1,1'-dioxide) has been reported from the same species.[20] Dipyrithione can be prepared in a laboratory by oxidation of pyrithione with chlorine in the presence of sodium hydroxide:[16]
- 2 C
5H
4NOSH + Cl
2 + 2 NaOH → ONC
5H
4–S–S–C
5H
4NO + 2 NaCl + 2 H
2O
- 2 C
Dipyrithione is used as a fungicide and bactericide,[8] and has been reported to possess novel cytotoxic activity by inducing apoptosis.[21] However, as apoptosis only occurs in higher organisms, this mechanism isn't relevant to the antifungal and bacteric idal properties of pyrithione.
Properties edit
(thione form on the left, thiolate form on the right)
Pyrithione exists as a pair of prototropes, a form of tautomerism whereby the rapid interconversion of constitutional isomers involves the shift of a single proton, in this case between the sulfur and oxygen atoms (shown in the infobox above).[3][22][23]
Salts of the conjugate base of pyrithione can also be considered to exhibit tautomerism by notionally associating the sodium ion with whichever heteroatom bears the negative charge of the anion (as opposed to the formal charges associated with the N-oxide); however, considering the anion alone, this could also be described as an example of resonance.
Pyrithione is a weak acid with pKa values of −1.95 and +4.6 (thiol proton),[2][3] but is a markedly stronger acid than either of its parent compounds (pyridine-N-oxide and pyridine-2-thiol), both of which have pKa > 8.[22] It is only slightly soluble in water (2.5 g L−1) but is soluble in many organic solvents (including benzene, chloroform, dichloromethane, dimethylformamide, dimethylsulfoxide, and ethyl acetate) and slight solubility in others (diethyl ether, ethanol, methyl tert-butyl ether, and tetrahydrofuran).[1]
Pyrithione can be used as a source of hydroxyl radical in organic synthesis[24] as it photochemically decomposes to HO• and (pyridin-2-yl)sulfanyl radical.[25]
Applications edit
Top: Structural formula of the monomer
Bottom: Ball-and-stick model of the dimer
The conjugate base of pyrithione (pyrithionate ion) is an anion containing two donor atoms, a sulfur atom and an oxygen atom each bearing a negative formal charge; the nitrogen atom remains formally positively charged. The thiolate anion can be formed by reaction with sodium carbonate, and zinc pyrithione is formed when zinc chloride is added.[10] The anion can act as either a monodentate or bidentate ligand and forms a 1:2 complex with a zinc(II) metal centre. Zinc pyrithione has been used since the 1930s though its preparation was not disclosed until a 1955 British patent[13] in which pyrithione was reacted directly with hydrated zinc sulfate in ethanol.[9] In its monomeric form, zinc pyrithione has two of the anions chelated to a zinc centre with a tetrahedral geometry. In the solid state, it forms a dimer in which each zinc centre adopts a trigonal bipyramidal geometry with two of the anions acting as bridging ligands coordinated through the oxygen atoms in the axial positions.[26] In solution, the dimers dissociate via scission of zinc-oxygen bonds to each bridging ligand. Further dissociation of the monomer into its constituents can occur and is undesirable as the complex is more potent in medical applications; for this reason, zinc carbonate can be added to formulations as it inhibits the monomer dissociation.[27]
Zinc pyrithione has a long history of use in medicated shampoos to treat dandruff and seborrhoeic dermatitis[28][29][30] (dandruff can be considered a mild form of seborrheic dermatitis[12]). It exhibits both antifungal and antimicrobial properties, inhibiting the Malassezia yeasts which promote these scalp conditions.[27] The mechanisms by which this work are the subject of ongoing study.[31][32] It can be used as an antibacterial agent against Staphylococcus and Streptococcus infections for conditions such as athlete's foot, eczema, psoriasis, and ringworm.[13] It is known to be cytotoxic against Pityrosporum ovale, especially in combination with ketoconazole, which is the preferred formulation for seborrheic dermatitis.[11] Pyrithione itself inhibits membrane transport processes in fungi.[22][33]
Paints used in external environments sometimes include zinc pyrithione as a preventive against algae and mildew.[13][34]
References edit
- ^ a b c d e f g h Knight, David W.; Hartung, Jens (15 September 2006). "1-Hydroxypyridine-2(1H)-thione". 1-Hydroxypyridine-2(1H)-thione. Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X.rh067.pub2. ISBN 0471936235.
- ^ a b Rodríguez Mellado, José Miguel; Marín Galvín, Rafael; Ruiz Montoya, Mercedes (2004). "Anthropogenic Pollutants of the Environment: Electrochemical Studies on Herbicides and Fungicides". In Brillas Coso, Enric; Cabot Julia, Pere-Lluís (eds.). Trends in Electrochemistry and Corrosion at the Beginning of the 21st Century: Dedicated to Professor Dr. Josep M. Costa on the Occasion of His 70th Birthday. Edicions Universitat Barcelona. pp. 335–358. ISBN 9788447526390. Archived from the original on 2024-02-24. Retrieved 2024-02-24.
- ^ a b c Jones, R. Alan; Katritzky, A. R. (1960). "N-oxides and related compounds. Part XVII. The tautomerism of mercapto- and acylamino-pyridine 1-oxides". J. Chem. Soc.: 2937–2942. doi:10.1039/JR9600002937.
- ^ a b Ebrahimia, R.; Zamani, Z.; Kash, A. (2009). "Genetic diversity evaluation of wild Persian shallot (Allium hirtifolium Boiss.) using morphological and RAPD markers". Sci. Hortic. 119 (4): 345–351. doi:10.1016/j.scienta.2008.08.032.
- ^ Bond, Andrew; Jones, William (1999). "1-Hydroxy-2(1H)-pyridinethione". Acta Crystallogr. C. 55 (9): 1536–1538. doi:10.1107/S0108270199006824.
- ^ a b Räth, C.; Binz, A.; Räth, C. (1931). "Mercaptane und Sulfosäuren des Pyridins. XII. Mitteilung über Derivate des Pyridins". Justus Liebigs Ann. Chem. (in German). 487: 105–119. doi:10.1002/jlac.19314870107.
- ^ a b Jones, R. A.; Katritzky, A. R. (1958). "721. Tautomeric pyridines. Part I. Pyrid-2- and −4-thione". J. Chem. Soc.: 3610–3613. doi:10.1039/JR9580003610.
- ^ a b c d Entry on Pyrithion. at: Römpp Online. Georg Thieme Verlag, retrieved 15 December 2016.
- ^ a b US granted 2809971, Bernstein, Jack & Losee, Kathryn A., "Heavy-metal derivatives of 1-hydroxy-2-pyridinethiones and method of preparing same", published 1957-10-15, assigned to Olin Mathieson Archived 2016-12-24 at the Wayback Machine
- ^ a b US granted 4396766, Farmer, David A. & Katz, Lawrence E., "Process for producing sodium and zinc pyrithione", published 1983-08-02, assigned to Olin Corporation Archived 2016-11-21 at the Wayback Machine
- ^ a b Gupta, Mrinal; Mahajan, Vikram K.; Mehta, Karaninder S.; Chauhan, Pushpinder S. (2014). "Zinc Therapy in Dermatology: A Review". Dermatol. Res. Pract. 2014: 1–11. doi:10.1155/2014/709152. PMC 4120804. PMID 25120566.
- ^ a b Chernoff, Karen; Lin, Richie; Cohen, Steven R. (2014). "Seborrheic Dermatitis". In Rudikoff, Donald; Cohen, Steven R.; Scheinfeld, Noah (eds.). Atopic Dermatitis and Eczematous Disorders. CRC Press. pp. 275–288. ISBN 9781840766530. Archived from the original on 2024-02-24. Retrieved 2024-02-24.
- ^ a b c d e "Molecule of the Week: Zinc pyrithione". American Chemical Society. February 10, 2014. Archived from the original on 1 March 2021. Retrieved 23 April 2020.
- ^ Shaw, Elliott; Bernstein, Jack; Losee, Kathryn; Lott, W. A. (1950). "Analogs of Aspergillic Acid. IV. Substituted 2-Bromopyridine-N-oxides and Their Conversion to Cyclic Thiohydroxamic Acids". J. Am. Chem. Soc. 72 (10): 4362–4364. doi:10.1021/ja01166a008.
- ^ a b Cheng, Hefeng; She, Ji (1990). "14. Improved preparation of 2-mercaptopyridine-N-oxide". Zhongguo Yiyao Gongye Zazhi. 21 (2): 55–56.
- ^ a b Unger, Thomas A. (1996). "Dipyrithione". Pesticide Synthesis Handbook. Noyes Publications. p. 853. ISBN 9780815518532. Archived from the original on 2024-02-24. Retrieved 2024-02-24.
- ^ Thomas, K.; Jerchel, D. (1964). "The Introduction of Substituents into the Pyridine Ring". In Foerst, Wilhelm (ed.). Newer Methods of Preparative Organic Chemistry. Vol. 3. Translated by Birnbaum, Henry. Academic Press. pp. 53–110. ISBN 9780323146104. Archived from the original on 2024-02-24. Retrieved 2024-02-24.
- ^ Mosher, H. S.; Turner, L.; Carlsmith, A. (1963). "Pyridine-N-oxide". Organic Syntheses. doi:10.15227/orgsyn.033.0079; Collected Volumes, vol. 4, p. 828.
- ^ Block, Eric; Dane, A. John; Cody, Robert B. (2011). "Crushing Garlic and Slicing Onions: Detection of Sulfenic Acids and Other Reactive Organosulfur Intermediates from Garlic and Other Alliums using Direct Analysis in Real-Time Mass Spectrometry (DART-MS)". Phosphorus Sulfur. 186 (5): 1085–1093. doi:10.1080/10426507.2010.507728. S2CID 98520689.
- ^ O'Donnell, Gemma; Poeschl, Rosemarie; Zimhony, Oren; Gunaratnam, Mekala; Moreira, Joao B. C.; Neidle, Stephen; Evangelopoulos, Dimitrios; Bhakta⊥, Sanjib; Malkinson, John P.; Boshoff, Helena I.; Lenaerts, Anne; Gibbons, Simon (2009). "Bioactive Pyridine-N-oxide Disulfides from Allium stipitatum". J. Nat. Prod. 72 (3): 360–365. doi:10.1021/np800572r. PMC 2765505. PMID 19093848.
- ^ Fan, Yumei; Liu, Caizhi; Huang, Yongmao; Zhang, Jie; Cai, Linlin; Wang, Shengnan; Zhang, Yongze; Duan, Xianglin; Yin, Zhimin (2013). "Dipyrithione induces cell-cycle arrest and apoptosis in four cancer cell lines in vitro and inhibits tumor growth in a mouse model". BMC Pharmacol. Toxicol. 14 (54): 54. doi:10.1186/2050-6511-14-54. PMC 4015681. PMID 24139500.
- ^ a b c Chandler, Carol J.; Segel, Irwin H. (1978). "Mechanism of the Antimicrobial Action of Pyrithione: Effects on Membrane Transport, ATP levels, and Protein Synthesis". Antimicrob. Agents Chemother. 14 (1): 60–68. doi:10.1128/AAC.14.1.60. PMC 352405. PMID 28693.
- ^ Katritzky, Alan R.; Elguero, José (1976). The Tautomerism of Heterocycles. Academic Press. ISBN 9780120206513.
- ^ Smith, Michael B. (2013). March's Advanced Organic Chemistry (7th ed.). Wiley. p. 246. ISBN 978-0-470-46259-1.
- ^ DeMatteo, Matthew P.; Poole, James S.; Shi, Xiaofeng; Sachdeva, Rakesh; Hatcher, Patrick G.; Hadad, Christopher M.; Platz, Matthew S. (2005). "On the Electrophilicity of Hydroxyl Radical: A Laser Flash Photolysis and Computational Study". Journal of the American Chemical Society. 127 (19): 7094–7109. doi:10.1021/ja043692q. ISSN 0002-7863. PMID 15884952.
- ^ Barnett, B. L.; Kretschmar, H. C.; Hartman, F. A. (1977). "Structural characterization of bis(N-oxopyridine-2-thionato)zinc(II)". Inorg. Chem. 16 (8): 1834–1838. doi:10.1021/ic50174a002.
- ^ a b Trüeb, Ralph M.; Lee, Won-Soo (2014). "6.2.5 – Dandruff". Male Alopecia: Guide to Successful Management. Springer Science & Business Media. pp. 247–250. ISBN 9783319032337. Archived from the original on 2024-02-24. Retrieved 2024-02-24.
- ^ Marks, R.; Pearse, A. D.; Walker, A. P. (1985). "The Effects of a Shampoo Containing Zinc Pyrithione on the Control of Dandruff". Br. J. Dermatol. 112 (4): 415–422. doi:10.1111/j.1365-2133.1985.tb02314.x. PMID 3158327. S2CID 23368244.
- ^ Faergemann, Jan (2000). "Management of Seborrheic Dermatitis and Pityriasis Versicolor". Am. J. Clin. Dermatol. 1 (2): 75–80. doi:10.2165/00128071-200001020-00001. PMID 11702314. S2CID 43516330.
- ^ Bacon, Robert A.; Mizoguchi, Haruko; Schwartz, James R. (2014). "Assessing therapeutic effectiveness of scalp treatments for dandruff and seborrheic dermatitis, part 1: A reliable and relevant method based on the adherent scalp flaking score (ASFS)". J. Dermatolog. Treat. 25 (3): 232–236. doi:10.3109/09546634.2012.687089. PMID 22515728. S2CID 30707098.
- ^ Chandler, Carol J.; Segel, Irwin H. (1978). "Mechanism of the Antimicrobial Action of Pyrithione: Effects on Membrane Transport, ATP Levels, and Protein Synthesis". Antimicrob. Agents Chemother. 14 (1): 60–68. doi:10.1128/AAC.14.1.60. PMC 352405. PMID 28693.
- ^ Reeder, N. L.; Xu, J.; Youngquist, R. S.; Schwartz, James R.; Rust, R. C.; Saunders, C. W. (2011). "The Antifungal Mechanism of Action of Zinc Pyrithione". Br. J. Dermatol. 165 (s2): 9–12. doi:10.1111/j.1365-2133.2011.10571.x. PMID 21919897. S2CID 31243048.
- ^ Ermolayeva, Elena; Sanders, Dale (1995). "Mechanism of Pyrithione-Induced Membrane Depolarization in Neurospora crassa". Appl. Environ. Microbiol. 61 (9): 3385–3390. Bibcode:1995ApEnM..61.3385E. doi:10.1128/AEM.61.9.3385-3390.1995. PMC 167618. PMID 7574648.
- ^ US patent 4039312, Joseph, Marcel & Patru, Gaston, "Bacteriostatic, fungistatic and algicidal compositions, particularly for submarine paints", published 1977-08-02, assigned to Joseph, Marcel and Patru, Gaston Archived 2017-02-02 at the Wayback Machine


