
KNOWPIA
WELCOME TO KNOWPIA
Summary
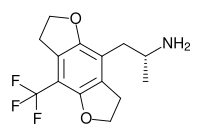
TFMFly is a compound related to psychedelic phenethylamines such as 2C-B-FLY and 2C-TFM. It was first reported in 2005 by a team at Purdue University led by David Nichols.[1] It acts as a potent agonist at the 5HT2A serotonin receptor subtype, and is a chiral compound with the more active (R) enantiomer having a Ki of 0.12 nM at the human 5-HT2A receptor.[2] While the fully aromatic benzodifurans such as Bromo-DragonFLY generally have higher binding affinity than saturated compounds like 2C-B-FLY,[3] the saturated compounds have higher efficacy as agonists.[4]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID |
|
| ChemSpider |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) |
|
| Chemical and physical data | |
| Formula | C14H16F3NO2 |
| Molar mass | 287.282 g·mol−1 |
| 3D model (JSmol) |
|
| |
| |
| (verify) | |
Legal Status edit
TFMFly is illegal in Latvia.[5]
See also edit
References edit
- ^ Braden MR (2007). Towards a biophysical understanding of hallucinogen action (Ph.D. thesis). Purdue University. ProQuest 304838368.
- ^ Parrish JC, Braden MR, Gundy E, Nichols DE (December 2005). "Differential phospholipase C activation by phenylalkylamine serotonin 5-HT 2A receptor agonists". Journal of Neurochemistry. 95 (6): 1575–84. doi:10.1111/j.1471-4159.2005.03477.x. PMID 16277614. S2CID 24005602.
- ^ Chambers JJ, Kurrasch-Orbaugh DM, Parker MA, Nichols DE (March 2001). "Enantiospecific synthesis and pharmacological evaluation of a series of super-potent, conformationally restricted 5-HT(2A/2C) receptor agonists". Journal of Medicinal Chemistry. 44 (6): 1003–10. CiteSeerX 10.1.1.691.362. doi:10.1021/jm000491y. PMID 11300881.
- ^ Heim R. Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur. Entwicklung eines neuen Struktur-Wirkungskonzepts (Ph.D. thesis) (in German). Freien Universität Berlin.
- ^ "Noteikumi par Latvijā kontrolējamajām narkotiskajām vielām, psihotropajām vielām un prekursoriem" [Regulations on Narcotic Drugs, Psychotropic Substances and Precursors Controlled in Latvia] (in Latvian). Ministry of Health of the Republic of Latvia.


