Summary
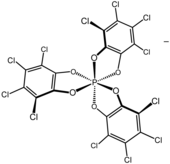
TRISPHAT (full name tris(tetrachlorocatecholato)phosphate(1−)) is an inorganic anion with the formula P(O
2C
6Cl
4)−
3 often prepared as the tributylammonium ((C
4H
9)
3NH+
) or tetrabutylammonium ((C
4H
9)
4N+
salt. The anion features phosphorus(V) bonded to three tetrachlorocatecholate (C
6Cl
4O2−
2) ligands. This anion can be resolved into the axially chiral enantiomers, which are optically stable (the picture shows the Δ enantiomer).
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Tetrabutylammonium tris(3,4,5,6-tetrachlorobenzene-1,2-diolato-κ2O1,O2)phosphorus(V)
| |||
| Other names
Tetrabutylammonium tris(tetrachlorocatecholato)phosphorus(1−)
Bu4N+ PHAT− 1-Butanaminium, N,N,N-tributyl-, (OC-6-11-Δ)-tris[3,4,5,6-tetrachloro-1,2-benzenediolato(2-)-κO1,κO2]phosphate(1−) (1:1) | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
| ||
| ChemSpider |
| ||
| ECHA InfoCard | 100.164.647 | ||
| EC Number |
| ||
PubChem CID
|
| ||
CompTox Dashboard (EPA)
|
| ||
| |||
| |||
| Properties | |||
| [C16H36N][C18Cl12O6P] | |||
| Molar mass | 1011.06 | ||
| Appearance | colourless solid | ||
| CH2Cl2 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |||
The TRISPHAT anion has been used as a chiral shift reagent for cations.[1] It improves the resolution of 1H NMR spectra by forming diastereomeric ion pairs.
Preparation edit
The anion is prepared by treatment of phosphorus pentachloride with tetrachlorocatechol followed by a tertiary amine gives the anion:
- PCl5 + 3 C6Cl4(OH)2 → H[P(O2C6Cl4)3] + 5 HCl
- H[P(O2C6Cl4)3] + Bu3N → Bu3NH+ [P(O2C6Cl4)3]−
Using a chiral amine, the anion can be readily resolved.[2]
References edit
- ^ Ratni, Hassen; Jodry, Jonathan J.; Lacour, Jérôme; Kündig, E. Peter (2000). "[n-Bu4N][Δ-TRISPHAT] Salt, an Efficient NMR Chiral Shift Reagent for Neutral Planar Chiral Tricarbonylchromium Complexes". Organometallics. 19 (19): 3997–3999. doi:10.1021/om000437f.
- ^ Favarger, France; Goujon-Ginglinger, Catherine; Monchaud, David; Lacour, Jérôme (2004). "Large-Scale Synthesis and Resolution of TRISPHAT [Tris(tetrachlorobenzenediolato) Phosphate(V)] Anion". Journal of Organic Chemistry. 69 (24): 8521–8524. doi:10.1021/jo048641q. PMID 15549835..