
Summary
The thalamus (pl.: thalami; from Greek θάλαμος, "chamber")[1] is a large mass of gray matter on the lateral walls of the third ventricle forming the dorsal part of the diencephalon (a division of the forebrain). Nerve fibers project out of the thalamus to the cerebral cortex in all directions, known as the thalamocortical radiations, allowing hub-like exchanges of information. It has several functions, such as the relaying of sensory and motor signals to the cerebral cortex[2][3] and the regulation of consciousness, sleep, and alertness.[4]
| Thalamus | |
|---|---|
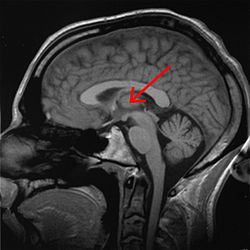 Thalamus marked (MRI cross-section) | |
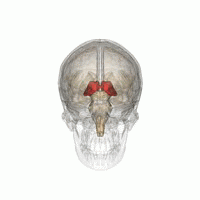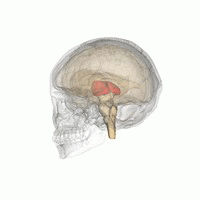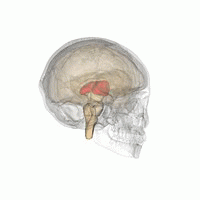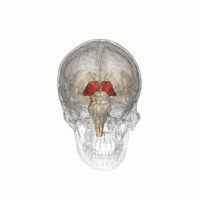 The thalamus in a 360° rotation | |
| Details | |
| Part of | Diencephalon |
| Parts | See List of thalamic nuclei |
| Artery | Posterior cerebral artery and branches |
| Identifiers | |
| Latin | thalamus dorsalis |
| MeSH | D013788 |
| NeuroNames | 300 |
| NeuroLex ID | birnlex_954 |
| TA98 | A14.1.08.101 A14.1.08.601 |
| TA2 | 5678 |
| TE | E5.14.3.4.2.1.8 |
| FMA | 62007 |
| Anatomical terms of neuroanatomy [edit on Wikidata] | |
Anatomically, it is a paramedian symmetrical structure of two halves (left and right), within the vertebrate brain, situated between the cerebral cortex and the midbrain. It forms during embryonic development as the main product of the diencephalon, as first recognized by the Swiss embryologist and anatomist Wilhelm His Sr. in 1893.[5]
Anatomy edit
The thalamus is a paired structure of gray matter about four centimetres long, located in the forebrain which is superior to the midbrain, near the center of the brain with nerve fibers projecting out to the cerebral cortex in all directions. The medial surface of the thalamus constitutes the upper part of the lateral wall of the third ventricle, and is connected to the corresponding surface of the opposite thalamus by a flattened gray band, the interthalamic adhesion. The lateral part of the thalamus is the phylogenetically newest part of the thalamus (neothalamus), and includes the lateral nuclei, the pulvinar and the medial and lateral geniculate nuclei.[6][7] There are areas of white matter in the thalamus including the stratum zonale that covers the dorsal surface and the external and internal medullary laminae. The external lamina covers the lateral surface and the internal lamina divides the nuclei into anterior, medial, and lateral groups.[8]
Blood supply edit
The thalamus derives its blood supply from a number of arteries: the polar artery (posterior communicating artery), paramedian thalamic-subthalamic arteries, inferolateral (thalamogeniculate) arteries, and posterior (medial and lateral) choroidal arteries.[9] These are all branches of the posterior cerebral artery.[10]
Some people have the artery of Percheron, which is a rare anatomic variation in which a single arterial trunk arises from the posterior cerebral artery to supply both parts of the thalamus.
Thalamic nuclei edit
Derivatives of the diencephalon include the dorsally-located epithalamus (essentially the habenula and annexes) and the perithalamus (prethalamus) containing the zona incerta and the thalamic reticular nucleus. Due to their different ontogenetic origins, the epithalamus and the perithalamus are formally distinguished from the thalamus proper. The metathalamus is made up of the lateral geniculate and medial geniculate nuclei.
The thalamus comprises a system of lamellae (made up of myelinated fibers) separating different thalamic subparts. Other areas are defined by distinct clusters of neurons, such as the periventricular nucleus, the intralaminar elements, the "nucleus limitans", and others.[11] These latter structures, different in structure from the major part of the thalamus, have been grouped together into the allothalamus as opposed to the isothalamus.[12] This distinction simplifies the global description of the thalamus.
Connections edit
The thalamus has many connections to the hippocampus via the mammillothalamic tract. This tract comprises the mammillary bodies and fornix.[13]
The thalamus is connected to the cerebral cortex via the thalamocortical radiations.[14]
The spinothalamic tract is a sensory pathway originating in the spinal cord. It transmits information to the thalamus about pain, temperature, itch and crude touch. There are two main parts: the lateral spinothalamic tract, which transmits pain and temperature, and the anterior (or ventral) spinothalamic tract, which transmits crude touch and pressure.
Function edit
The thalamus has multiple functions, and is generally believed to act as a relay station, or hub, relaying information between different subcortical areas and the cerebral cortex.[15] In particular, every sensory system (with the exception of the olfactory system) includes a thalamic nucleus that receives sensory signals and sends them to the associated primary cortical area.[citation needed] For the visual system, for example, inputs from the retina are sent to the lateral geniculate nucleus of the thalamus, which in turn projects to the visual cortex in the occipital lobe.[16] The thalamus is believed to both process sensory information as well as relay it—each of the primary sensory relay areas receives strong feedback connections from the cerebral cortex.[17] Similarly the medial geniculate nucleus acts as a key auditory relay between the inferior colliculus of the midbrain and the primary auditory cortex.[citation needed] The ventral posterior nucleus is a key somatosensory relay, which sends touch and proprioceptive information to the primary somatosensory cortex.[citation needed]
The thalamus also plays an important role in regulating states of sleep and wakefulness.[18] Thalamic nuclei have strong reciprocal connections with the cerebral cortex, forming thalamo-cortico-thalamic circuits that are believed to be involved with consciousness.[19] The thalamus plays a major role in regulating arousal, the level of awareness, and activity. Damage to the thalamus can lead to permanent coma.[20]
The role of the thalamus in the more anterior pallidal and nigral territories in the basal ganglia system disturbances is recognized but still poorly understood. The contribution of the thalamus to vestibular or to tectal functions is almost ignored. The thalamus has been thought of as a "relay" that simply forwards signals to the cerebral cortex. Newer research suggests that thalamic function is more selective.[21] Many different functions are linked to various regions of the thalamus. This is the case for many of the sensory systems (except for the olfactory system), such as the auditory, somatic, visceral, gustatory and visual systems where localized lesions provoke specific sensory deficits. A major role of the thalamus is support of motor and language systems, and much of the circuitry implicated for these systems is shared. The thalamus is functionally connected to the hippocampus[22] as part of the extended hippocampal system at the thalamic anterior nuclei[23] with respect to spatial memory and spatial sensory datum they are crucial for human episodic event memory.[24][25] The thalamic region's connection to the mesio-temporal lobe provide differentiation of the functioning of recollective and familiarity memory.[13]
The neuronal information processes necessary for motor control were proposed as a network involving the thalamus as a subcortical motor center.[26] Through investigations of the anatomy of the brains of primates[27] the nature of the interconnected tissues of the cerebellum to the multiple motor cortices suggested that the thalamus fulfills a key function in providing the specific channels from the basal ganglia and cerebellum to the cortical motor areas.[28][29] In an investigation of the saccade and antisaccade[30] motor response in three monkeys, the thalamic regions were found to be involved in the generation of antisaccade eye-movement (that is, the ability to inhibit the reflexive jerking movement of the eyes in the direction of a presented stimulus).[31]
Recent research suggests that the mediodorsal thalamus (MD) may play a broader role in cognition. Specifically, the mediodorsal thalamus may "amplify the connectivity (signaling strength) of just the circuits in the cortex appropriate for the current context and thereby contribute to the flexibility (of the mammalian brain) to make complex decisions by wiring the many associations on which decisions depend into weakly connected cortical circuits."[32] Researchers found that "enhancing MD activity magnified the ability of mice to "think,"[32] driving down by more than 25 percent their error rate in deciding which conflicting sensory stimuli to follow to find the reward."[33]
Development edit
The thalamic complex is composed of the perithalamus (or prethalamus, previously also known as ventral thalamus), the mid-diencephalic organiser (which forms later the zona limitans intrathalamica (ZLI) ) and the thalamus (dorsal thalamus).[34][35] The development of the thalamus can be subdivided into three steps.[36] The thalamus is the largest structure deriving from the embryonic diencephalon, the posterior part of the forebrain situated between the midbrain and the cerebrum.
Early brain development edit
After neurulation the anlage of the prethalamus and the thalamus is induced within the neural tube. Data from different vertebrate model organisms support a model in which the interaction between two transcription factors, Fez and Otx, are of decisive importance. Fez is expressed in the prethalamus, and functional experiments show that Fez is required for prethalamus formation.[37][38] Posteriorly, Otx1 and Otx2 abut the expression domain of Fez and are required for proper development of the thalamus.[39][40]
Formation of progenitor domains edit
Early in thalamic development two progenitor domains form, a caudal domain, and a rostral domain. The caudal domain gives rise to all of the glutamatergic neurons in the adult thalamus while the rostral domain gives rise to all of the GABAergic neurons in the adult thalamus.[41]
The formation of the mid-diencephalic organiser (MDO) edit
At the interface between the expression domains of Fez and Otx, the mid-diencephalic organizer (MDO, also called the ZLI organiser) is induced within the thalamic anlage. The MDO is the central signalling organizer in the thalamus. A lack of the organizer leads to the absence of the thalamus. The MDO matures from ventral to dorsal during development. Members of the sonic hedgehog (SHH) family and of the Wnt family are the main principal signals emitted by the MDO.
Besides its importance as signalling center, the organizer matures into the morphological structure of the zona limitans intrathalamica (ZLI).
Maturation and parcellation of the thalamus edit
After its induction, the MDO starts to orchestrate the development of the thalamic anlage by release of signalling molecules such as SHH.[42] In mice, the function of signaling at the MDO has not been addressed directly due to a complete absence of the diencephalon in SHH mutants.[43]
Studies in chicks have shown that SHH is both necessary and sufficient for thalamic gene induction.[44] In zebrafish, it was shown that the expression of two SHH genes, SHH-a and SHH-b (formerly described as twhh) mark the MDO territory, and that SHH signaling is sufficient for the molecular differentiation of both the prethalamus and the thalamus but is not required for their maintenance and SHH signaling from the MDO/alar plate is sufficient for the maturation of prethalamic and thalamic territory while ventral Shh signals are dispensable.[45]
The exposure to SHH leads to differentiation of thalamic neurons. SHH signaling from the MDO induces a posterior-to-anterior wave of expression the proneural gene Neurogenin1 in the major (caudal) part of the thalamus, and Ascl1 (formerly Mash1) in the remaining narrow stripe of rostral thalamic cells immediately adjacent to the MDO, and in the prethalamus.[46][47]
This zonation of proneural gene expression leads to the differentiation of glutamatergic relay neurons from the Neurogenin1+ precursors and of GABAergic inhibitory neurons from the Ascl1+ precursors. In fish, selection of these alternative neurotransmitter fates is controlled by the dynamic expression of Her6 the homolog of HES1. Expression of this hairy-like bHLH transcription factor, which represses Neurogenin but is required for Ascl1, is progressively lost from the caudal thalamus but maintained in the prethalamus and in the stripe of rostral thalamic cells. In addition, studies on chick and mice have shown that blocking the Shh pathway leads to absence of the rostral thalamus and substantial decrease of the caudal thalamus. The rostral thalamus will give rise to the reticular nucleus mainly whereby the caudal thalamus will form the relay thalamus and will be further subdivided in the thalamic nuclei.[36]
In humans, a common genetic variation in the promoter region of the serotonin transporter (the SERT-long and -short allele: 5-HTTLPR) has been shown to affect the development of several regions of the thalamus in adults. People who inherit two short alleles (SERT-ss) have more neurons and a larger volume in the pulvinar and possibly the limbic regions of the thalamus. Enlargement of the thalamus provides an anatomical basis for why people who inherit two SERT-ss alleles are more vulnerable to major depression, post-traumatic stress disorder, and suicide.[48]
Clinical significance edit
A thalamus damaged by a stroke can lead to thalamic pain syndrome,[49] which involves a one-sided burning or aching sensation often accompanied by mood swings. Bilateral ischemia of the area supplied by the paramedian artery can cause serious problems including akinetic mutism, and be accompanied by oculomotor problems. A related concept is thalamocortical dysrhythmia. The occlusion of the artery of Percheron can lead to a bilateral thalamus infarction.
Alcoholic Korsakoff syndrome stems from damage to the mammillary body, the mammillothalamic fasciculus or the thalamus.[50][51]
Fatal familial insomnia is a hereditary prion disease in which degeneration of the thalamus occurs, causing the patient to gradually lose their ability to sleep and progressing to a state of total insomnia, which invariably leads to death. In contrast, damage to the thalamus can result in coma.
Additional images edit
-
Human brain dissection, showing the thalamus.
-
Human thalamus along with other subcortical structures, in glass brain.
-
Lateral group of the thalamic nuclei.
-
Medial group of the thalamic nuclei.
See also edit
- 5-HT7 receptor
- Krista and Tatiana Hogan - conjoined twins with joined thalami
- List of regions in the human brain
- Nonmotor region of the ventral nuclear group of the thalamus
- Nucleus ventralis posterior lateralis pars oralis (VPLo), a region of the thalamus
- Primate basal ganglia system
- Thalamic stimulator
- Thalamotomy
References edit
- ^ Harper - index Archived 2015-05-12 at the Wayback Machine & University of Washington Faculty Web Server Archived 2015-03-02 at the Wayback Machine & Search engine search page Archived 2020-09-27 at the Wayback Machine + Perseus Project tufts.edu Archived 2021-03-05 at the Wayback Machine Retrieved 2012-02-09
- ^ Sherman, S. (2006). "Thalamus". Scholarpedia. 1 (9): 1583. Bibcode:2006SchpJ...1.1583S. doi:10.4249/scholarpedia.1583.
- ^ Sherman, S. Murray; Guillery, R. W. (2000). Exploring the Thalamus. Academic Press. pp. 1–18. ISBN 978-0-12-305460-9.
- ^ Gorvett, Zaria. "What you can learn from Einstein's quirky habits". bbc.com.
- ^ Jones, Edward G, ed. (1985). The Thalamus - Springer. doi:10.1007/978-1-4615-1749-8. ISBN 978-1-4613-5704-9. S2CID 41337319.
- ^ "Medical Definition of NEOTHALAMUS". www.merriam-webster.com.
- ^ "neothalamus | Definition of neothalamus in English by Oxford Dictionaries". Oxford Dictionaries | English. Archived from the original on May 27, 2018.
- ^ Tortora, Gerard; Anagnostakos, Nicholas (1987). Principles of anatomy and physiology (5th. Harper international ed.). New York: Harper & Row. p. 314. ISBN 978-0060466695.
- ^ Percheron, G. (1982). "The arterial supply of the thalamus". In Schaltenbrand; Walker, A. E. (eds.). Stereotaxy of the human brain. Stuttgart: Thieme. pp. 218–32.
- ^ Knipe, H Jones, J et al. Thalamus http://radiopaedia.org/articles/thalamus Archived 2017-09-17 at the Wayback Machine
- ^ Jones Edward G. (2007) "The Thalamus" Cambridge Uni. Press[page needed]
- ^ Percheron, G. (2003). "Thalamus". In Paxinos, G.; May, J. (eds.). The human nervous system (2nd ed.). Amsterdam: Elsevier. pp. 592–675.
- ^ a b Carlesimo, GA; Lombardi, MG; Caltagirone, C (2011). "Vascular thalamic amnesia: A reappraisal". Neuropsychologia. 49 (5): 777–89. doi:10.1016/j.neuropsychologia.2011.01.026. PMID 21255590. S2CID 22002872.
- ^ University of Washington (1991). "Thalamocortical radiations". washington.edu.
- ^ Gazzaniga; Ivry; Mangun, Michael, S.; Richard B.; George R. (2014). Cognitive Neuroscience - The Biology of The Mind. New York: W.W. Norton. pp. 45. ISBN 978-0-393-91348-4.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Shin, Lisa M; Liberzon, Israel (January 2010). "The Neurocircuitry of Fear, Stress, and Anxiety Disorders". Neuropsychopharmacology. 35 (1): 169–191. doi:10.1038/npp.2009.83. PMC 3055419.
- ^ "The thalamus, middleman of the brain, becomes a sensory conductor". The University of Chicago Medicine. Retrieved 10 September 2020.
- ^ Steriade, Mircea; Llinás, Rodolfo R. (1988). "The Functional States of the Thalamus and the Associated Neuronal Interplay". Physiological Reviews. 68 (3): 649–742. doi:10.1152/physrev.1988.68.3.649. PMID 2839857.
- ^ Coma and Disorders of Consciousness ISBN 978-1-447-12439-9 p. 143
- ^ The Neurology of Consciousness: Cognitive Neuroscience and Neuropathology ISBN 978-0-123-74168-4 p. 10
- ^ Leonard, Abigail W. (August 17, 2006). "Your Brain Boots Up Like a Computer". LiveScience.
- ^ Stein, Thor; Moritz, Chad; Quigley, Michelle; Cordes, Dietmar; Haughton, Victor; Meyerand, Elizabeth (2000). "Functional Connectivity in the Thalamus and Hippocampus Studied with Functional MR Imaging". American Journal of Neuroradiology. 21 (8): 1397–401. PMC 7974059. PMID 11003270.
- ^ Aggleton, John P.; Brown, Malcolm W. (1999). "Episodic memory, amnesia, and the hippocampal–anterior thalamic axis" (PDF). Behavioral and Brain Sciences. 22 (3): 425–44, discussion 444–89. doi:10.1017/S0140525X99002034. PMID 11301518. S2CID 11258997.
- ^ Aggleton, John P.; O'Mara, Shane M.; Vann, Seralynne D.; Wright, Nick F.; Tsanov, Marian; Erichsen, Jonathan T. (2010). "Hippocampal-anterior thalamic pathways for memory: Uncovering a network of direct and indirect actions". European Journal of Neuroscience. 31 (12): 2292–307. doi:10.1111/j.1460-9568.2010.07251.x. PMC 2936113. PMID 20550571.
- ^ Burgess, Neil; Maguire, Eleanor A; O'Keefe, John (2002). "The Human Hippocampus and Spatial and Episodic Memory". Neuron. 35 (4): 625–41. doi:10.1016/S0896-6273(02)00830-9. PMID 12194864. S2CID 11989085.
- ^ Evarts, E V; Thach, W T (1969). "Motor Mechanisms of the CNS: Cerebrocerebellar Interrelations". Annual Review of Physiology. 31: 451–98. doi:10.1146/annurev.ph.31.030169.002315. PMID 4885774.
- ^ Orioli, PJ; Strick, PL (1989). "Cerebellar connections with the motor cortex and the arcuate premotor area: An analysis employing retrograde transneuronal transport of WGA-HRP". The Journal of Comparative Neurology. 288 (4): 612–26. doi:10.1002/cne.902880408. PMID 2478593. S2CID 27155579.
- ^ Asanuma C, Thach WT, Jones EG (May 1983). "Cytoarchitectonic delineation of the ventral lateral thalamic region in the monkey". Brain Research. 286 (3): 219–35. doi:10.1016/0165-0173(83)90014-0. PMID 6850357. S2CID 25013002.
- ^ Kurata, K (2005). "Activity properties and location of neurons in the motor thalamus that project to the cortical motor areas in monkeys". Journal of Neurophysiology. 94 (1): 550–66. doi:10.1152/jn.01034.2004. PMID 15703228.
- ^ "The Antisaccade - A Review of Basic Research and Clinical Studies". Archived from the original on 2017-09-16. Retrieved 2012-02-10.[full citation needed]
- ^ Kunimatsu, J; Tanaka, M (2010). "Roles of the primate motor thalamus in the generation of antisaccades" (PDF). Journal of Neuroscience. 30 (14): 5108–17. doi:10.1523/JNEUROSCI.0406-10.2010. PMC 6632795. PMID 20371831.
- ^ a b "New Role Discovered For Brain Region". Neuroscience News. 2017-05-03. Retrieved 2017-12-03.
- ^ Schmitt, L. Ian; Wimmer, Ralf D.; Nakajima, Miho; Happ, Michael; Mofakham, Sima; Halassa, Michael M. (May 2017). "Thalamic amplification of cortical connectivity sustains attentional control". Nature. 545 (7653): 219–223. Bibcode:2017Natur.545..219S. doi:10.1038/nature22073. PMC 5570520. PMID 28467827.
- ^ Kuhlenbeck, Hartwig (1937). "The ontogenetic development of the diencephalic centers in a bird's brain (chick) and comparison with the reptilian and mammalian diencephalon". The Journal of Comparative Neurology. 66: 23–75. doi:10.1002/cne.900660103. S2CID 86730019.
- ^ Shimamura, K; Hartigan, DJ; Martinez, S; Puelles, L; Rubenstein, JL (1995). "Longitudinal organization of the anterior neural plate and neural tube". Development. 121 (12): 3923–33. doi:10.1242/dev.121.12.3923. PMID 8575293.
- ^ a b Scholpp, Steffen; Lumsden, Andrew (2010). "Building a bridal chamber: Development of the thalamus". Trends in Neurosciences. 33 (8): 373–80. doi:10.1016/j.tins.2010.05.003. PMC 2954313. PMID 20541814.
- ^ Hirata, T.; Nakazawa, M; Muraoka, O; Nakayama, R; Suda, Y; Hibi, M (2006). "Zinc-finger genes Fez and Fez-like function in the establishment of diencephalon subdivisions". Development. 133 (20): 3993–4004. doi:10.1242/dev.02585. PMID 16971467.
- ^ Jeong, J.-Y.; Einhorn, Z.; Mathur, P.; Chen, L.; Lee, S.; Kawakami, K.; Guo, S. (2007). "Patterning the zebrafish diencephalon by the conserved zinc-finger protein Fezl". Development. 134 (1): 127–36. doi:10.1242/dev.02705. PMID 17164418.
- ^ Acampora, D; Avantaggiato, V; Tuorto, F; Simeone, A (1997). "Genetic control of brain morphogenesis through Otx gene dosage requirement". Development. 124 (18): 3639–50. doi:10.1242/dev.124.18.3639. PMID 9342056.
- ^ Scholpp, S.; Foucher, I.; Staudt, N.; Peukert, D.; Lumsden, A.; Houart, C. (2007). "Otx1l, Otx2 and Irx1b establish and position the ZLI in the diencephalon". Development. 134 (17): 3167–76. doi:10.1242/dev.001461. PMC 7116068. PMID 17670791.
- ^ Song, Hobeom; Lee, Bumwhee; Pyun, Dohoon; Guimera, Jordi; Son, Youngsook; Yoon, Jaeseung; Baek, Kwanghee; Wurst, Wolfgang; Jeong, Yongsu (February 2015). "Ascl1 and Helt act combinatorially to specify thalamic neuronal identity by repressing Dlxs activation". Developmental Biology. 398 (2): 280–291. doi:10.1016/j.ydbio.2014.12.003. PMID 25512300.
- ^ Puelles, L; Rubenstein, JL (2003). "Forebrain gene expression domains and the evolving prosomeric model". Trends in Neurosciences. 26 (9): 469–76. doi:10.1016/S0166-2236(03)00234-0. PMID 12948657. S2CID 14658562.
- ^ Ishibashi, M; McMahon, AP (2002). "A sonic hedgehog-dependent signaling relay regulates growth of diencephalic and mesencephalic primordia in the early mouse embryo". Development. 129 (20): 4807–19. doi:10.1242/dev.129.20.4807. PMID 12361972.
- ^ Kiecker, C; Lumsden, A (2004). "Hedgehog signaling from the ZLI regulates diencephalic regional identity". Nature Neuroscience. 7 (11): 1242–9. doi:10.1038/nn1338. PMID 15494730. S2CID 29863625.
- ^ Scholpp, S.; Wolf, O; Brand, M; Lumsden, A (2006). "Hedgehog signalling from the zona limitans intrathalamica orchestrates patterning of the zebrafish diencephalon". Development. 133 (5): 855–64. doi:10.1242/dev.02248. PMID 16452095.
- ^ Scholpp, S.; Delogu, A.; Gilthorpe, J.; Peukert, D.; Schindler, S.; Lumsden, A. (2009). "Her6 regulates the neurogenetic gradient and neuronal identity in the thalamus". Proceedings of the National Academy of Sciences. 106 (47): 19895–900. Bibcode:2009PNAS..10619895S. doi:10.1073/pnas.0910894106. PMC 2775703. PMID 19903880.
- ^ Vue, Tou Yia; Bluske, Krista; Alishahi, Amin; Yang, Lin Lin; Koyano-Nakagawa, Naoko; Novitch, Bennett; Nakagawa, Yasushi (2009). "Sonic Hedgehog Signaling Controls Thalamic Progenitor Identity and Nuclei Specification in Mice". Journal of Neuroscience. 29 (14): 4484–97. doi:10.1523/JNEUROSCI.0656-09.2009. PMC 2718849. PMID 19357274.
- ^ Young, Keith A.; Holcomb, Leigh A.; Bonkale, Willy L.; Hicks, Paul B.; Yazdani, Umar; German, Dwight C. (2007). "5HTTLPR Polymorphism and Enlargement of the Pulvinar: Unlocking the Backdoor to the Limbic System". Biological Psychiatry. 61 (6): 813–8. doi:10.1016/j.biopsych.2006.08.047. PMID 17083920. S2CID 2214561.
- ^ Dejerine, J.; Roussy, G. (1906). "Le syndrome thalamique". Revue Neurologique. 14: 521–32.
- ^ Kopelman, MD; Thomson, AD; Guerrini, I; Marshall, EJ (16 January 2009). "The Korsakoff syndrome: clinical aspects, psychology and treatment". Alcohol and Alcoholism. 44 (2): 148–54. doi:10.1093/alcalc/agn118. PMID 19151162.
- ^ Rahme, R; Moussa, R; Awada, A; Ibrahim, I; Ali, Y; Maarrawi, J; Rizk, T; Nohra, G; Okais, N; Samaha, E (April 2007). "Acute Korsakoff-like amnestic syndrome resulting from left thalamic infarction following a right hippocampal hemorrhage". AJNR. American Journal of Neuroradiology. 28 (4): 759–60. PMC 7977335. PMID 17416834.
- ^ Wexler, Marisa (20 October 2023). "Machine learning models estimate when brain atrophy starts in MS". Multiple Sclerosis News Today.
- ^ Cen, Steven; Gebregziabher, Mulugeta; Moazami, Saeed; Azevedo, Christina J.; Pelletier, Daniel (28 September 2023). "Toward precision medicine using a "digital twin" approach: modeling the onset of disease-specific brain atrophy in individuals with multiple sclerosis". Scientific Reports. 13 (1): 16279. Bibcode:2023NatSR..1316279C. doi:10.1038/s41598-023-43618-5. PMC 10187410.
External links edit
- Stained brain slice images which include the "thalamus" at the BrainMaps project


