
KNOWPIA
WELCOME TO KNOWPIA
Summary
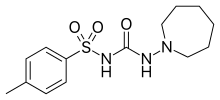
Tolazamide is an oral blood glucose lowering drug used for people with Type 2 diabetes. It is part of the sulfonylurea family (ATC A10BB).
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Tolinase |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682482 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | metabolized in the liver to active metabolites |
| Elimination half-life | 7 hours |
| Excretion | Renal (85%) and fecal (7%) |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID |
|
| IUPHAR/BPS |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) |
|
| ECHA InfoCard | 100.013.262 |
| Chemical and physical data | |
| Formula | C14H21N3O3S |
| Molar mass | 311.40 g·mol−1 |
| 3D model (JSmol) |
|
| |
| |
| (verify) | |
Synthesis edit
para-Toluenesulfonamide is converted to its carbamate with ethyl chloroformate in the presence of a base. Heating that intermediate with 1-amino-azepane leads to the displacement of the ethoxy group and the formation of tolazemide:[1]
Azepane proper would lead to [13078-23-4].
References edit
External links edit
- "Tolazamide". Medline Plus. U.S. National Library of Medicine.


