
Summary
Vanadium(V) oxytrifluoride is a chemical compound with the formula VOF3. It is one of several vanadium(V) oxyhalides. VOF3 is a yellowish orange powder that is sensitive to moisture.[2] Characteristic of early metal fluorides, the structure is polymeric in the solid state. The solid adopts a layered structure but upon evaporation, the species becomes dimeric. In contrast VOCl3 and VOBr3 remain tetrahedral in all states, being volatile liquids at room temperature.[3]
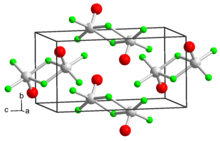
| |
| Names | |
|---|---|
| Other names
Vanadium oxyfluoride, trifluorooxovanadium
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider |
|
| ECHA InfoCard | 100.033.849 |
PubChem CID
|
|
| |
| Properties | |
| F3OV | |
| Molar mass | 123.9599 g/mol |
| Appearance | yellowish orange powder |
| Density | 2.4590 g/cm3 |
| Melting point | 300 °C (572 °F; 573 K) |
| Boiling point | 480 °C (896 °F; 753 K) |
| insoluble | |
| Hazards | |
| GHS labelling:[1] | |
  
| |
| Danger | |
| H302, H312, H314, H332 | |
| P260, P261, P264, P270, P271, P280, P301+P310, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P322, P330, P361, P363, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related compounds
|
VF5 VOCl3 VO2F |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Reactions edit
In organic synthesis, VOF3 is used for the oxidative coupling of phenols, for example in the syntheses of vancomycin and its analogues.[4] For these applications VOF3 is typically dissolved in trifluoroacetic acid.
Vanadium(V) oxytrifluoride reacts with hexamethyldisiloxane to give vanadium dioxide fluoride:[5]
- (CH3)3SiOSi(CH3)3 + VOF3 → VO2F + 2 (CH3)3SiF
References edit
- ^ "Trifluorooxovanadium". pubchem.ncbi.nlm.nih.gov.
- ^ Perry, Dale L. (2011). Handbook of Inorganic Compounds. CRC Press. ISBN 978-1-4398-1461-1.
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Vanasse, Benoit; O'Brien, Michael K. (2001). "Vanadyl Trifluoride". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rv005. ISBN 0471936235.
- ^ Davis, Martin F.; Jura, Marek; Leung, Alethea; Levason, William; Littlefield, Benjamin; Reid, Gillian; Webster, Michael (2008). "Synthesis, Chemistry and Structures of Complexes of the Dioxovanadium(v) Halides VO2F and VO2Cl". Dalton Transactions (44): 6265–6273. doi:10.1039/b811422f. PMID 18985260.



