
Summary
Zineb is the chemical compound with the formula {Zn[S2CN(H)CH2CH2N(H)CS2]}n. Structurally, it is classified as a coordination polymer and a dithiocarbamate complex. This pale yellow solid is used as fungicide.[2]
| |
| Names | |
|---|---|
| IUPAC name
zinc ethane-1,2-diylbis(dithiocarbamate)
| |
| Other names
1,2 ethanediylbis[dithiocarbamodithioato](2−) zinc,
Dithane Z-78, Aphytora, Amitan | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| 4165797 | |
| ChEBI |
|
| ChemSpider |
|
| ECHA InfoCard | 100.031.970 |
| EC Number |
|
| KEGG |
|
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
| UN number | 2771 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H6N2S4Zn | |
| Molar mass | 275.8 g/mol (monomer) |
| Appearance | pale yellow powder |
| Hazards[1] | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
skin sensitizer |
| GHS labelling: | |

| |
| Warning | |
| H317, H335 | |
| P261, P271, P280, P302+P352, P304+P340, P312, P333+P313, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Production and applications edit
It is produced by treating ethylene bis(dithiocarbamate) sodium salt, "nabam", with zinc sulfate. This procedure can be carried out by mixing nabam and zinc sulfate in a spray tank.[3] Its uses include control of downy mildews, rusts, and redfire disease.[2] In the US it was once registered as a "General Use Pesticide", however all registrations were voluntarily cancelled following an EPA special review.[3] It continues to be used in many other countries.
Structure edit
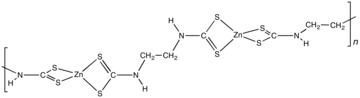
Zineb is a polymeric complex of zinc with a dithiocarbamate.[2] The polymer is composed of Zn(dithiocarbamate)2 subunits linked by an ethylene (-CH2CH2-) backbone.[4] A reference compound is [Zn(S2CNEt2)2]2, which features a pair of tetrahedral Zn centers bridged by one sulfur center.[5]
See also edit
- Metam sodium - A related dithiocarbamate salt which is also used as a fungicide.
- Maneb - ethylene bis(dithiocarbamate) with manganese instead of zinc.
- Mancozeb - A common fungicide containing Zineb and Maneb.
References edit
- ^ "Zinc, [[2-[(dithiocarboxy)amino]ethyl]carbamodithioato(2-)-kappaS,kappaS']-". pubchem.ncbi.nlm.nih.gov.
- ^ a b c Franz Müller; Peter Ackermann; Paul Margot (2012). "Fungicides, Agricultural, 2. Individual Fungicides". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.o12_o06. ISBN 978-3527306732.
- ^ a b Michael A. Kamrin, (1997) Pesticide Profiles: Toxicity, Environmental Impact, and Fate, CRC Press, ISBN 1-56670-190-2[page needed]
- ^ R. Engst; W. Schnaak (1974). "Residues of dithiocarbamate fungicides and their metabolites on plant foods". In Gunther F.A. (ed.). Residue Reviews. Vol. 52. New York, NY: Springer. pp. 45–46. doi:10.1007/978-1-4615-8504-6_3. ISBN 978-1-4615-8506-0.
- ^ Bonamico, M.; Mazzone, G.; Vaciago, A.; Zambonelli, L. (1965). "Structural studies of metal dithiocarbamates. III. The Crystal and Molecular Structure of Zinc Diethyldithiocarbamate". Acta Crystallogr. 19 (6): 898–909. doi:10.1107/S0365110X65004620.
External links edit
- Zineb in the Pesticide Properties DataBase (PPDB)


