
KNOWPIA
WELCOME TO KNOWPIA
Summary
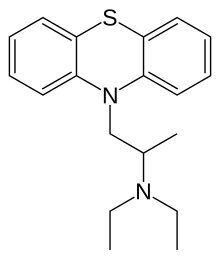
Profenamine (INN; also known as ethopropazine (BAN); solde under the trade name Parsidol and others) is a phenothiazine derivative used as an antiparkinsonian agent[1][2] that has anticholinergic, antihistamine, and antiadrenergic actions. It is also used in the alleviation of the extrapyramidal syndrome induced by drugs such as other phenothiazine compounds, but, like other compounds with antimuscarinic properties, is of no value against tardive dyskinesia.
 | |
| Clinical data | |
|---|---|
| Trade names | Parsidol, Parsidan, Parkisol, Parkin |
| AHFS/Drugs.com | International Drug Names |
| ATC code |
|
| Pharmacokinetic data | |
| Protein binding | 93% |
| Elimination half-life | 1 to 2 hours |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID |
|
| IUPHAR/BPS |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) |
|
| ECHA InfoCard | 100.007.566 |
| Chemical and physical data | |
| Formula | C19H24N2S |
| Molar mass | 312.48 g·mol−1 |
| 3D model (JSmol) |
|
| |
| |
| | |
Synthesis edit
For promoting bone growth:[3]
The alkylation between phenothiazine [92-84-2] (1) and 1-Diethylamino-2-chloropropane [761-21-7] (2) in the presence of Sodium amide gives ethopropazine (3).
- The aziridinium salt helps to rationalize why a rearrangement product is observed (ala methadone). This was also observewd for Aceprometazine.
References edit
- ^ "Prefenamine". drugs.com.
- ^ Morton IK, Hall JM (1999). "Ethopropazine". Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Dordrecht: Springer Netherlands. p. 115. ISBN 9789401144391.
- ^ Debra Ellies, William Rosenberg, WO 2010025135 (2010 to Osteogenex Inc.).
- ^ Charpentier, P. et al, Compt. Rend., 1951, 232, 415.
- ^ P. Carpentier, U.S. patent 2,526,118 (1950 to Societe desusines Chimiqiues).
- ^ Julius Nicholson Ashley, U.S. patent 2,607,773 (1952 to Societe des Bsines Chhniques Rhone).


